Active Metals Lab Practical
advertisement

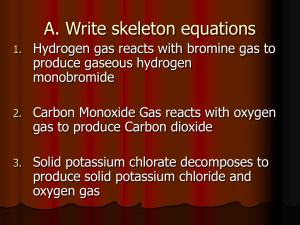
Active Metals Lab Practical I) Purpose: you are going to react Na, K, Al, Mg and Ca with 100-200ml de-ionized water and observe the intensity(violence) and duration(time) of the reaction. Using this information and the three factors that affect ionization energy you will explain the trends in the 5 element block. II) Theory: Central to this lab is the interplay between 1) Shielding, 2) Nuclear Charge and 3) Full and ½ Full level stability. Nuclear charge increases left to right. Shielding is less for Na, Mg and Al, more for K and Ca. Mg and Ca both have full S sublevel stability. All reactions follow these patterns: 2Na(s) + 2H2O(l) 2NaOH(aq) + H2(g) or Ca(s) + 2H2O(l) Ca(OH)2(aq) + H2(g) or 2Al(s) + 6H2O 2Al(OH)3 + 3H2(g) Completed reaction should have a very High pH(10+) due to the extreme basicity of all hydroxides. Use pH paper provided by Welch to determine this after all reactions are complete. All complete reactions can go down the sink after. Incomplete or no reaction need to have solid residue in the wastebasket. Have fun!