Determination of the Molar Mass of a Volatile Compound using the
advertisement

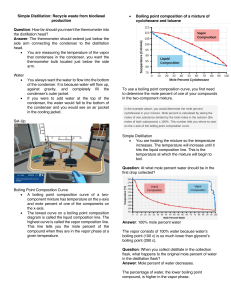
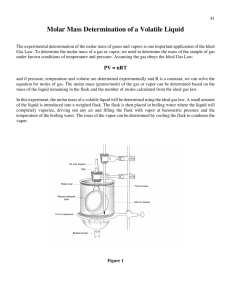
Determination of the Molar Mass of a Volatile Compound using the Dumas Method • At fixed temperature and moles, V = constant x 1/P. As pressure increases volume decreases. • At fixed pressure and moles, V = constant x T. Volume of gas will increase as temperature increases. • At fixed pressure and temperature, V = constant x n. Volume of gas will increase as the mole increases. • When all above is combined V= constant x 1/P x T x n Or PV = constant x n x T Let constant = R, then PV= nRT the Ideal Gas Law Standard pressure and temperature are 1 atm and 273o K respectively. One mole of gas occupies 22.4 liter at STP. Using the equation below the constant, R can be calculated at STP PV = R nT 1 atm x 22.4 L = 0.082057 L atm/K mole = R 1 mole x 273 K The Ideal Gas Law can be used to determine the molar mass of a volatile compound using the Dumas method. How? 1. Weigh a flask with a foil cover with a small pinhole and boiling chip. 2. Add approximately 5 mL of unknown sample to the flask. 3. Heat the sample in boiling water. The vapor that the flask can not contain is vented out through the pinhole. 4. The vapor that is contained in the flask is condensed and weighed. 5. Calculate the molecular weight of the unknown using the Ideal Gas Law. PV = nRT • P is the barometric pressure, V is the volume of the gas which is equal to the volume of the flask. • R is the constant 0.082057 L atm/K mole • T is the temperature of the boiling water which is equal to the temperature of the unknown’s vapor in Kelvin • “n” is the moles of the unknown gas that the flask held. 6. Molar mass of your unknown liquid is the mass (g) of the vapor (difference between the flask + foil + boiling chip + the condensed vapor and the empty flask + foil + boiling chip)/n (mole)