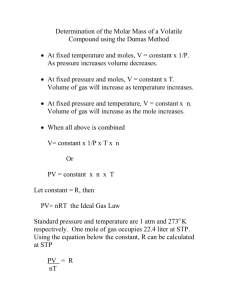
Lab 15 Molecular Mass of a Volatile liquid Tasnuva Israil 02/14/2012
advertisement

Lab 15 Molecular Mass of a Volatile liquid Tasnuva Israil 02/14/2012 1.) If the outside of your flask is not dry when the first mass determination is made and it is dried for the second mass determination, what effect will that have on your results? If the outside of the flask is not dry for the first mass determination and it is dried for the second mass determination this will affect my results. The mass of the aluminum cap and the empty will be greater and it will cause the molecular mass to decrease. 2.) What will be the effect on your results if all the liquid is not vaporized while heating? If all the liquid is not vaporized while heating that means the mass of the vapor is higher than the mass of the empty flask. That causes the molecular mass to increase. 3.) Which assumption would lead to the greatest error in your results, explain why? (A) assume temperature is 25 degress celcius (B) assume the flask volume is exactly 125 mL That will cause a greater error in my results because it can cause a different amount of liquid to be used which causes an inaccurate change in the colume used. Ultimately, that causes a great variable in the mass of the vapor used. (C) assume the barometric pressure is exactly 760.