(1) Statistical Interpretation of Entropy What is the physical origin of
advertisement
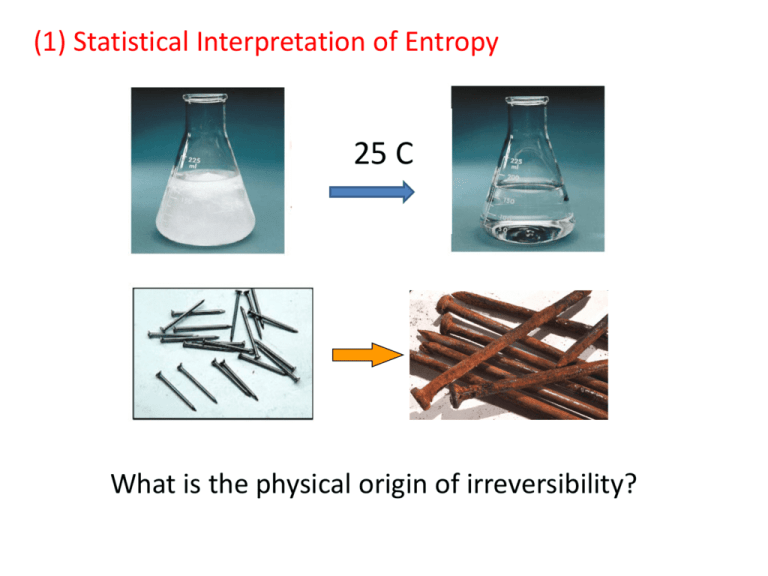
(1) Statistical Interpretation of Entropy 25 C What is the physical origin of irreversibility? Example: Free expansion of gas Left: Vacuum, Right: Full of gas Gas will be evenly distributed You will not see the reverse process in billion years! Total N particles. Same volume in each side. Question: How many different ways are possible to have NL particles on the left and NR particles on the right (N = NL + NR)? * Example: N=4, NL = 3, NR = 1 4 possible “microstates” In general, for a system of N particles with NL particles on the left and NR particles on the right, the number of possible microstates, W(NL,NR), is given by the following formula. N! W(NL,NR) = NL! NR! Check: N=4, NL = 3, NR = 1 W(3,1) = 4! 3! 1! =4 * Question: How does W(NL,NR) look like as the total number of particles increases? N=4 W(NL,NR) NL =4, NR=0 NL =3, NR=1 NL =2, NR=2 NL =1, NR=3 NL =0, NR=4 1 4 6 4 1 Probability (completely even) = 6/16 = 3/8 Probability (completely uneven) = 2/16 = 1/8 With 4 particles, we will often see the case where all particles are on the same side. N= 4 N=10 N=100 If N=1000, W(500,500) = 2.5 × 10299 Conclusion: As N -> 1023 , any departure from even distribution (equilibrium) is virtually impossible. Once the system reaches an equilibrium, there will be no apparent change in the system. (1) In free gas expansion example, once the valve opens, NL changes from its original value (NL=N) to NL = N/2, which maximizes the W(NL,NR) . (2) Isolated system moves to the “direction” that maximize W. (3) W is related to the directionality of process, which can be measured in terms of “entropy” . S = kB ln W (Boltzmann, ~1890) kB= 1.38 J/K “Boltzmann Constant” (4) NL reaches a value that maximizes the entropy of the system (isolated). (5) Entropy Change: dS dSTOTAL > 0 : Irreversible Change dSTOTAL = 0 : Equilibrium