Stoichiometry Worksheet 1 Answer Key
advertisement
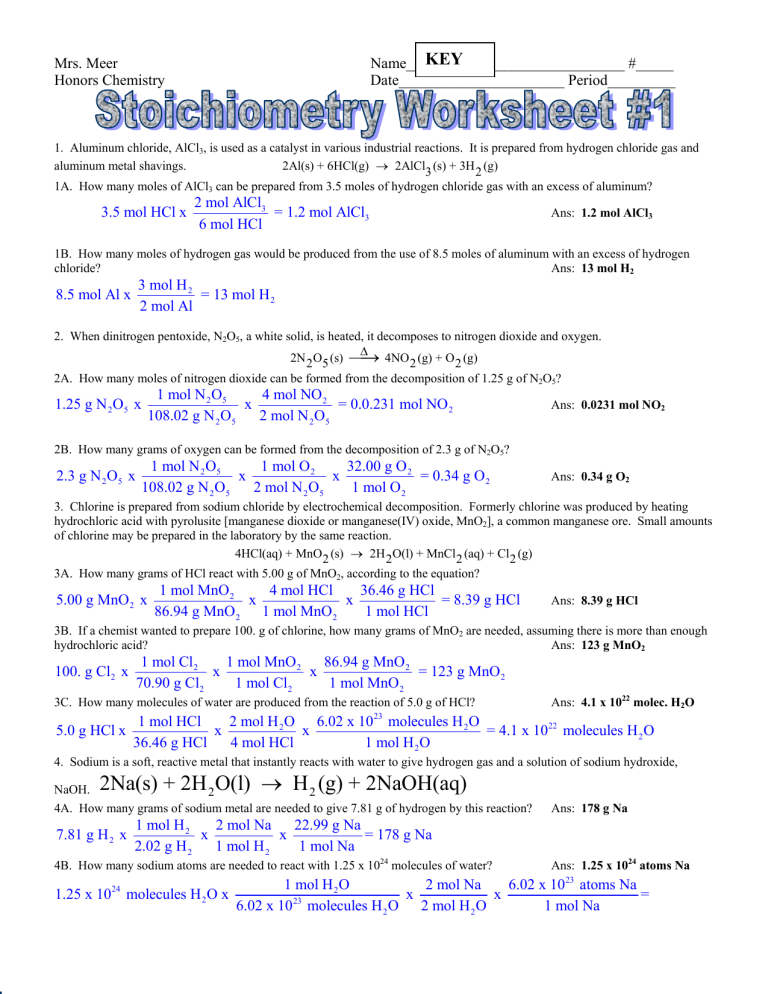
KEY Name_____________________________ #_____ Date______________________ Period_________ Mrs. Meer Honors Chemistry 1. Aluminum chloride, AlCl3, is used as a catalyst in various industrial reactions. It is prepared from hydrogen chloride gas and aluminum metal shavings. 2Al(s) + 6HCl(g) → 2AlCl3 (s) + 3H 2 (g) 1A. How many moles of AlCl3 can be prepared from 3.5 moles of hydrogen chloride gas with an excess of aluminum? 3.5 mol HCl x 2 mol AlCl3 = 1.2 mol AlCl3 6 mol HCl Ans: 1.2 mol AlCl3 1B. How many moles of hydrogen gas would be produced from the use of 8.5 moles of aluminum with an excess of hydrogen chloride? Ans: 13 mol H2 3 mol H 2 = 13 mol H 2 2 mol Al 8.5 mol Al x 2. When dinitrogen pentoxide, N2O5, a white solid, is heated, it decomposes to nitrogen dioxide and oxygen. Δ 2N 2 O5 (s) ⎯⎯ → 4NO 2 (g) + O 2 (g) 2A. How many moles of nitrogen dioxide can be formed from the decomposition of 1.25 g of N2O5? 1.25 g N 2 O5 x 1 mol N 2 O5 4 mol NO 2 x = 0.0.231 mol NO 2 108.02 g N 2 O5 2 mol N 2 O5 Ans: 0.0231 mol NO2 2B. How many grams of oxygen can be formed from the decomposition of 2.3 g of N2O5? 2.3 g N 2O5 x 1 mol N 2 O5 1 mol O 2 32.00 g O 2 x x = 0.34 g O 2 108.02 g N 2 O5 2 mol N 2 O5 1 mol O 2 Ans: 0.34 g O2 3. Chlorine is prepared from sodium chloride by electrochemical decomposition. Formerly chlorine was produced by heating hydrochloric acid with pyrolusite [manganese dioxide or manganese(IV) oxide, MnO2], a common manganese ore. Small amounts of chlorine may be prepared in the laboratory by the same reaction. 4HCl(aq) + MnO 2 (s) → 2H 2 O(l) + MnCl 2 (aq) + Cl 2 (g) 3A. How many grams of HCl react with 5.00 g of MnO2, according to the equation? 5.00 g MnO 2 x 1 mol MnO 2 4 mol HCl 36.46 g HCl x x = 8.39 g HCl 86.94 g MnO 2 1 mol MnO 2 1 mol HCl Ans: 8.39 g HCl 3B. If a chemist wanted to prepare 100. g of chlorine, how many grams of MnO2 are needed, assuming there is more than enough hydrochloric acid? Ans: 123 g MnO2 100. g Cl2 x 1 mol Cl2 1 mol MnO 2 86.94 g MnO 2 x x = 123 g MnO 2 70.90 g Cl2 1 mol Cl2 1 mol MnO 2 3C. How many molecules of water are produced from the reaction of 5.0 g of HCl? 5.0 g HCl x Ans: 4.1 x 1022 molec. H2O 2 mol H 2 O 6.02 x 1023 molecules H 2 O 1 mol HCl x x = 4.1 x 1022 molecules H 2 O 36.46 g HCl 4 mol HCl 1 mol H 2 O 4. Sodium is a soft, reactive metal that instantly reacts with water to give hydrogen gas and a solution of sodium hydroxide, NaOH. 2Na(s) + 2H 2 O(l) → H 2 (g) + 2NaOH(aq) 4A. How many grams of sodium metal are needed to give 7.81 g of hydrogen by this reaction? 7.81 g H 2 x Ans: 178 g Na 1 mol H 2 2 mol Na 22.99 g Na x x = 178 g Na 2.02 g H 2 1 mol H 2 1 mol Na 4B. How many sodium atoms are needed to react with 1.25 x 1024 molecules of water? Ans: 1.25 x 1024 atoms Na 1 mol H 2 O 2 mol Na 6.02 x 1023 atoms Na 1.25 x 10 molecules H 2 O x x x = 6.02 x 1023 molecules H 2 O 2 mol H 2 O 1 mol Na 24 Stoichiometry Worksheet #1 continued 5. Hematite, Fe2O3, is an important ore of iron. The free metal is obtained by reacting hematite with carbon monoxide in a blast furnace. Carbon monoxide if formed in the furnace by partial combustion of carbon. Fe 2 O3 (s) + 3CO(g) → 2Fe(s) + 3CO 2 (g) How many grams of iron can be produced from 1.00 kg Fe2O3? 1.00 kg Fe 2 O3 x Ans: 699 g Fe 1000 g Fe2 O3 1 mol Fe 2 O3 2 mol Fe 55.85 g Fe x x x = 699 g Fe 1 kg Fe2 O3 159.70 g Fe 2O3 1 mol Fe 2 O3 1 mol Fe 6. Sphalerite is a zinc sulfide (ZnS) mineral and an important commercial source of zinc metal. The first step in the processing of the ore consists of heating the sulfide with oxygen to give zinc oxide, ZnO, and sulfur dioxide, SO2. How many kilograms of oxygen gas combine with 5.00 x 103 g of zinc sulfide in this reaction? Ans: 2.46 kg O2 2ZnS(s) + 3O 2 (g) → 2ZnO(s) + 2SO 2 (g) 5.00 x 103 g ZnS x 1 mol ZnS 3 mol O 2 32.00 g O 2 1 kg O 2 x x x = 2.46 kg O 2 97.45 g ZnS 2 mol ZnS 1 mol O 2 1000 g O 2 7. The British chemist Joseph Priestley prepared oxygen in 1774 by heating mercury(II) oxide, HgO. Mercury metal is the other product. If 6.47 g of oxygen is collected, how many grams of mercury metal are also produced? Δ 2HgO(s) ⎯⎯ → 2Hg(s) + O 2 (g) 6.47 g O 2 x Ans: 81.1 g Hg 1 mol O 2 2 mol Hg 200.59 g Hg x x = 81.1 g Hg 32.00 g O 2 1 mol O 2 1 mol Hg 8. In a process for producing acetic acid, oxygen gas is bubbles into acetaldehyde, CH3CHO, containing manganese(II) acetate Mn(C2H3O2 )2 (s) (catalyst) under pressure at 60oC. 2CH3CHO(l) + O 2 (g) ⎯⎯⎯⎯⎯⎯⎯ → 2HC2 H3O 2 (l) If 20.0 g of acetaldehyde is reacted with an excess of oxygen, how many grams of acetic acid can be produced? Ans: 27.3 g HC2H3O2 20.0 g CH 3CHO x 1 mol CH 3CHO 2 mol HC2 H 3O 2 60.06 g HC2 H 3O 2 x x = 27.3 g HC2 H 3O 2 44.06 g CH 3CHO 2 mol CH 3CHO 1 mol HC2 H 3O 2 9. Some industrial plants for acetic acid react liquid methanol with carbon monoxide in the presence of a catalyst. CH3OH(l) + CO(g) → HC 2 H3O 2 (l) 3.23 x 1023 molecules of methanol were placed in a reaction vessel with an excess of carbon monoxide. How many grams of acetic acid can be produced? Ans: 32.2 g HC2H3O2 3.23 x 1023 molecules CH3OH x 1 mol CH 3OH 1 mol HC2 H 3O 2 60.06 g HC2 H 3O 2 x x = 23 6.02 x 10 molecules CH 3OH 1 mol CH 3OH 1 mol HC2 H3O2 10. Titanium dioxide [titanium(IV) oxide] is used as the base powder for a variety of cosmetics. Say you decide to manufacture Ti(s) + O2(g) → TiO2(s) TiO2 in a furnace by the reaction If a company wants to produce 3.0 kg of titanium dioxide, how many grams of titanium should be reacted? Ans: 1.8 x 103 g Ti 3.0 kg TiO 2 x 1000 g TiO 2 1 mol TiO 2 1 mol Ti 47.90 g Ti x x x = 1.8 x 103 g Ti 1 kg TiO 2 79.90 g TiO 2 1 mol TiO 2 1 mol Ti 11. Tungsten metal, W, is used to make incandescent bulb filaments. The metal is produced from the yellow tungsten(VI) oxide, WO3, by reaction with hydrogen. WO3 (s) + 3H 2 (g) → W(s) + 3H 2 O(g) How many grams of tungsten can be obtained from 1.4 x 1027 molecules of hydrogen with excess tungsten(VI) oxide? Ans: 1.4 x 105 g W 1.4 x 1027 molecules H 2 x 1 mol H 2 1 mol W 183.85 g W x x = 1.4 x 105 g W 23 6.02 x 10 molecules H 2 3 mol H 2 1 mol W 12. Potassium superoxide, KO2, is used in breathing gas masks to regenerate oxygen. 4KO 2 (s) + 2H 2 O(l) → 4KOH(s) + 3O 2 (g) If a reaction vessel contains 0.25 mol KO2, how many molecules of oxygen can be produced? 0.25 mol KO 2 x Ans: 1.1 x 1023 molec. O2 3 mol O 2 6.02 x 1023 molecules O 2 x = 1.1 x 1023 molecules O 2 4 mol KO 2 1 mol O 2









