Unit 2 Section A - Volume vs. Temperature: Charles' Law
advertisement

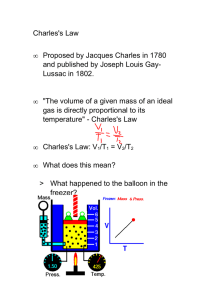
Name Unit 2 Section A - Volume vs. Temperature: Charles’ Law Use Charles’ law, which describes the temperature–volume relationship for a gas, to solve the following problems. Show your setup on each one. For some questions, it may be necessary to convert temperature(s) to kelvins. 1. The temperature of a gas is 0.0 °C and the temperature is changed so the gas volume increases from 21 L to 42 L. a) Was the temperature increased or decreased? _____________________________ What is the new temperature of the gas, in oC? Assume pressure does not change. V 1= V 2= T 1= T 2= Original Equation: Solution: Temperature = ___________________ 2. A gas has a volume of 10.0 m3 at standard temperature (273 K). What volume will the gas occupy if the kelvin temperature is doubled at constant pressure? V 1= V 2= T 1= T 2= Original Equation: Solution: Volume = ___________________ 3. Suppose 500.0 mL of oxygen is collected at 25 °C. What will be the volume if the temperature is increased to 50 °C and pressure remains constant? V 1= V 2= T 1= T 2= Original Equation: Solution: Volume = ___________________ 4. A gas occupies a volume of 560.0 cm3 at a temperature of 120 °C. In °C, to what temperature must the gas be lowered if it is to occupy 400.0 cm3 without a change in pressure? V 1= V 2= T 1= T 2= Original Equation: Solution: Temperature = ___________________ 5. Suppose 100.0 mL of nitrogen gas is collected at 21.0 °C. If the temperature of the gas is increased to 60.0 °C at constant pressure, what will be the new volume of the gas? V 1= V 2= T 1= T 2= Original Equation: Solution: Volume = ___________________ 6. A helium-filled balloon has a volume of 2.75 L at 20.0 °C. The volume of the balloon decreases to 2.46 L after it is taken outside on a winter day. In oC, what is the outside temperature? V 1= V 2= T 1= T 2= Original Equation: Solution: Temperature = ___________________