Spectroscopy in Astronomy: Emission Spectra Equipment: (Shared
advertisement

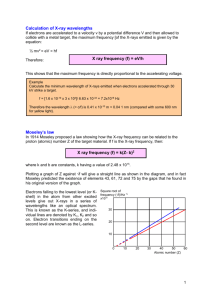
Spectroscopy in Astronomy: Emission Spectra Equipment: (Shared among all lab groups) Blue spectrometer (one for each lab group) Spectrum tube power supplies (5000 volts—be careful!) These will be set up around the room, one with a hydrogen gas tube, and three others with unknowns. Sodium light (up front) Red reading lamps, set up next to the discharge tubes. Your answers to questions, descriptions of your procedures, data (including tables), and results will need to be written up and handed in on a separate lab report. Make sure to include an overall discussion and conclusions for the lab. Introduction: A spectroscope is a device used by astronomers (and others) to separate light into its various color components. Basically, there are two types of spectroscopes: one uses a prism usually made of glass while the other uses a diffraction grating which is made of a plate of glass with very fine and accurately placed parallel scratches on one face. The grating or prism in a spectroscope is called the dispersing element. The purposes of this experiment are: (i) To familiarize yourself with the use of a very simple spectrograph, (ii) To determine how accurately you can measure wavelengths in an emission-line spectrum, and (iii) To identify some unknown gases from their spectra. Procedure: Divide yourselves into groups of 3 or 4. Over the course of the lab, make sure that everybody has a chance to use the spectrograph, and take some data. Begin by examining your spectrograph. It should look roughly like the following image, when viewed from above: scale slit eyepiece At the eyepiece end, what looks like a clear piece of plastic is actually a transmission diffraction grating, with thousands of grooves cut into it. Much of the light that hits the grating passes straight through, and you are able to see through it. Some of the light, however, hits the grooves and scatters in all directions. In certain angles, light coming from different grooves interferes constructively. This happens at different angles for different wavelengths, with the result that the grating spreads the light out into a spectrum. The process is sketched below, for two grooves (the gratings on the spectrographs actually have thousands of grooves). Bright points appear here where the difference in the paths between the observer and the two slits is an integer number of wavelengths. Planar light waves coming in. Screen with two slits Light scatters off of slits The calibration of the spectrographs is not perfect. Before proceeding, you’ll need to see how well calibrated yours is. First, look through the eyepiece of the spectrograph, while aiming at the room lights (everyone in your group should do this). You will see two scales. One gives photon energies in electron volts (eV), and the other gives photon wavelengths. 1) What are the numbers on the wavelength scale? Two units are commonly used when measuring the wavelengths of visible light. Most physicists in the world use nanometers (nm), where 1 nm = 10-9 m. The unit most commonly used by astronomers is the Ångstrom, where 1 Å = 10-10 m. 2) Based upon the numbers that you see on the scale, what units do you think that we will be using to measuring wavelengths in this lab? 3) What are the smallest divisions on the scale? How precisely do you believe that you will be able to make wavelength measurements using this scale (give your expected precision as a number)? Now, make sure that the slit is aimed at the overhead lights. Do you see a spectrum projected against the scale on the side? If not, then rotate the eyepiece that holds the diffraction grating until the spectrum of the room lights is projected on the scale. Make sure that everybody in your group is able to see the spectrum of the lights, and that you individually record the answer to the following question: 4) Looking at the overhead lights, you should see a continuous spectrum, with some brighter lines at certain wavelengths. Record the wavelengths of the brightest lines that you can see. Compare them to the location of the brightest lines of Mercury given below. How closely do they compare. Quantify your answer. That means to use numbers: “pretty close” doesn’t qualify as a comparison! Mercury Gas Color Wavelength (nm) Violet 405 Violet 436 Blue 492 Green 546 Yellow 577 Yellow 579 Orange 607 Red 691 Now make another check of the location of the scale, and learn to use the spectrograph in the dark. Once everybody has completed step 1, the room lights will be turned off, and the instructor will turn on the sodium light. Sodium emits a doublet of lines (two lines spaced very closely together), at 589.0 and 589.6 nm. These will appear as a single line in your spectrograph. (The pureness of the emission explains why things look so odd when illuminated by streetlights that use low pressure sodium gas.) 5) At what wavelength do you see the sodium doublet? What is the percentage difference between what you see and the actual average doublet wavelength of 589.3 nm? 6) Tabulate the difference between the wavelengths recorded on your spectrograph and the actual wavelengths. Fill in the following table for all the lines that you could see (transcribe it onto your final lab report). All units should be in nm. For the difference, subtract the true wavelength from your measurement (so, if your spectrograph reads wavelengths as being too small, the difference will be negative). For the ratio, divide the true wavelength by your measurement (so, if your spectrograph reads too large, the ratio will be less than one). For the final row, average the results of your differences and ratios. Element Hg Hg Hg Hg Hg Hg Hg Na Average Actual Wavelength (nm) Measured wavelength (nm) ------- ------- Difference Ratio 7) Go to the lab station with the power supply holding the hydrogen gas tube, and turn it on. In order to see the scale better, it will help to project the red reading lamp either at the wall or onto a sheet of paper such that the red light illuminates the scale of the spectrograph. Don’t shine the red lamp directly at the spectrograph—it’ll be too bright, and will swamp your spectrum. You will probably see 3 lines in your spectrograph. Complete the following table for the lines that you can see: True wavelength (nm) Color 656.3 486.1 434.1 410.2 Red Aqua Violet Violet Measured wavelength (nm) Correction 1 Correction 2 Which is better? For “Correction 1” take your measured wavelengths and subtract the average difference that you found in step 6. For “Correction 2” take your measured wavelengths and multiply by the average ratio that you found in step 6. In the final column, decide which of the two corrections gave you a wavelength that was closer to the true wavelength. Hopefully (but not certainly!) it will be the same for all of the lines. Don’t worry if it isn’t. Pick as your best the one that works better for more lines. 8) Now try to identify some unknown elements using their emission spectra. There are 3 or 4 unknown tubes at stations around the lab, labeled by number. Look at each of the unknowns with your spectroscope, write down how many lines you can see for each, and your best measurement of the wavelength of each one. Keep a record of these on a separate sheet of paper, and hand it in as part of the data section of your lab report. 9) As you work on step 8, make your best guess as to the identity of each of the unknown elements, and record them, along with the reasons for your choices. To do this, compare your results with the lists below, with charts in your text, the color charts that your instructor will have, the charts on the web page listed below, and with the chart in the lab room. Visual comparisons will be the easiest, and so the list of wavelengths is recommended only as a last resort. Hint: the unknowns will be from among helium (He), oxygen (O), nitrogen (N), neon (Ne), argon (Ar), xenon (Xe), and mercury (Hg). In making your comparisons, keep the following points in mind: a) You won’t see all of the lines that are either shown on the images or listed below—our spectrographs are too crude for that. b) If you see lines in your spectrograph that are either not listed or are not shown, then you have a bad match, and should look at another element. c) Don’t worry too much about the extreme red and violet wavelengths. Our eyes aren’t very sensitive to wavelengths near 400 nm and 700 nm, and so you may not see something there that appears on the list or on the images. This problem varies from person to person, but gets worse with age—see for example the images at the top of the web page, where the instructor is comparing his perception of the spectra with that of his students. d) If you are comparing the numbers that you measure with those on the lists below, keep in mind the differences and corrections that you found in steps 6 and 7 above. If you’d like some extra credit, tabulate your measured wavelengths, and include the best correction that you found in step 7. Wavelengths of some of the elements. All wavelengths listed below are in Ångstroms. Divide by 10 to get the wavelengths in nm. You will probably find it easier to use a visual representation, which can be found at http://astro.u-strasbg.fr/~koppen/discharge/. Your instructor will have a color version of this as well. Also look at the chart in the classroom, and at the chart that your instructor will have, which is based on the figures from the above web site. Helium (He) 4388 Å 4471 Å 4713 Å 4922 Å 5016 Å 5048 Å Argon (Ar) 4610 4658 6677 6753 5876Å 6678 Å 4727 6871 4736 6965 4765 4806 4880 4965 5142 6115 6172 Krypton (Kr) 4274 4320 4355 4847 4946 5022 5871 5992 6420 4470 5087 6456 4577 5126 6570 4619 5208 6904 4659 5309 4739 5333 4766 5468 4825 5570 4832 5682 6143 6929 Neon (Ne) 4569 5852 6164 6182 7032 5873 6217 5945 6267 5965 6334 5975 6383 5976 6402 6030 6507 6074 6599 Oxygen (O) 4593 4638 5436 5577 6653 4645 5958 4662 6046 4676 6106 4700 6157 4925 6261 4943 6370 5330 6454 Xenon (Xe) 4501 4525 4917 4923 5895 5931 6473 4583 5028 5934 4624 5393 6164 4671 5460 6178 4697 5696 6180 4734 5697 6182 4793 5716 6198 4807 5824 6201 4830 5825 6318 4843 5875 6470 Nitrogen (N) 3943 3998 4060 4574 4649 4667 4095 4724 4142 4815 4201 4917 4270 4976 4344 4355 4417 4490 Since nitrogen is a molecule, its spectrum consists of bands rather than lines. This is due to rotation of the molecules. In the visible part of the spectrum, the most prominent structure is the First Positive series, with about 30 regularly spaced bands in the region 5000-7000 Å. Only the band heads of the less intense Second Positive series are listed above. Prelab Questions: 1) Describe what is meant by constructive and destructive interference of light waves. 2) If two rays of light have a path length difference of 500 nm, what is their wavelength if they constructively interfere (show your work)? 3) What happens during the creation of the emission spectra which you observe from the discharge tubes? 4) (show your work, or describe how you arrive at your answers) a) If an electron is in the ground state of a hydrogen atom, what wavelength photon must be absorbed in order to promote the electron up to the third energy level? b) If an electron jumps from the third energy level down to the second, what wavelength photon is produced? What color will this photon appear as?