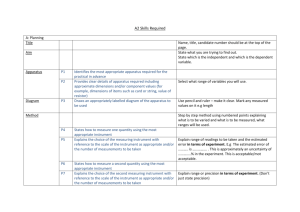
CHEMISTRY 11 UNCERTAINTY There is no such thing as a perfect
advertisement

CHEMISTRY 11 UNCERTAINTY There is no such thing as a perfect measurement. Each measurement contains a degree of uncertainty due to the limits of instruments and the people using them. In laboratory exercises, students are expected to follow the same procedure that scientists follow when they make measurements. Each measurement should be reported with some digits that are certain plus one digit with a value that has been estimated. UNCERTAINTY IN MEASUREMENTS eg. ruler graduated cylinder beaker scientific balance electronic balance The uncertainty is usually half of the marking on the glassware or equipment for eg. a grad cylinder marked every 1 ml would have an uncertainty of 0.5 ml On a digital instrument, the uncertainty or deviation or error is usually listed on the instrument. TWO MAIN CAUSES OF UNCERTAINTY IN THE LAB: 1. human error (in reading the measuring tool) 2. instrument uncertainty (inherent in its operation) HOW UNCERTAINTY RELATES TO YOUR CHEMISTRY LABWORK: 1. Lab data contains varying degrees of uncertainty 2. Graphing points includes some uncertainty as to the placement of coordinates 3. Calculations compound uncertainty in the various values used in a calculation Two concepts that have to do with measurements are accuracy and precision: ACCURACY The accuracy of the measurement refers to how close the measured value is to the true or accepted value. For example, if you used a balance to find the mass of a known standard 100.00 g mass, and you got a reading of 78.55 g, your measurement would not be very accurate. One important distinction between accuracy and precision is that accuracy can be determined by only one measurement, while precision can only be determined with multiple measurements. PRECISION Precision refers to how close together a group of measurements actually are to each other. Precision has nothing to do with the true or accepted value of a measurement, so it is quite possible to be very precise and totally inaccurate. In many cases, when precision is high and accuracy is low, the fault can lie with the instrument. If a balance or a thermometer is not working correctly, they might consistently give inaccurate answers, resulting in high precision and low accuracy. A dartboard analogy is often used to help students understand the difference between accuracy and precision. Imagine a person throwing darts, trying to hit the bull's-­‐eye. The closer the dart hits to the bull's-­‐eye, the more accurate his or her tosses are. If the person misses the dartboard with every throw, but all of their shots land close together, they can still be very precise. You must strive for both accuracy and precision in all of your laboratory activities this year. Make sure that you understand the workings of each instrument, take each measurement carefully, and recheck to make sure that you have precision. Without accurate and precise measurement your calculations, even if done correctly, are quite useless. THE SHORT ANSWER: ACCURACY -­‐how close you are to an accepted value -­‐IN GRAPHS: how close your average (or sometimes slope) is to the true value PRECISION -­‐how close your measurements are to each other -­‐refers to the uncertainty in the measurement IN GRAPHS: how close all your data points are to the best fit line SEE p. 75 in Heath: Figure at the bottom CHEMISTRY 11 UNCERTAINTY There is no such thing as a ______________________________. Each measurement contains a degree of ____________________ due to the limits of _______________________________. In laboratory exercises, students are expected to follow the same procedure that scientists follow when they make measurements. Each measurement should be reported with some digits that are _______________ plus one digit with a value that has been ________________________. UNCERTAINTY IN MEASUREMENTS eg. ruler graduated cylinder beaker scientific balance electronic balance The uncertainty is usually ______________________ on the glassware or equipment for eg. a grad cylinder marked every 1 ml would have an uncertainty of 0.5 ml On a digital instrument, the ______________________________________________ is usually listed on the instrument. TWO MAIN CAUSES OF UNCERTAINTY IN THE LAB: 1. ________________________________________________________________ 2. ________________________________________________________________ HOW UNCERTAINTY RELATES TO YOUR CHEMISTRY LABWORK: 1. ________________________________________________________________ 2. ________________________________________________________________ 3. ________________________________________________________________ Two concepts that have to do with measurements are accuracy and precision: ACCURACY The accuracy of the measurement refers to ________________________________ ____________________________________________________________________ For example, if you used a balance to find the mass of a known standard 100.00 g mass, and you got a reading of 78.55 g, your measurement would _______________ __________________________________. One important distinction between accuracy and precision is that _______________ ____________________________, while precision can only be determined ________ __________________________________. PRECISION Precision refers to _____________________________________________________ ________________. Precision has nothing to do with the __________________ of a measurement, so it is quite possible to be __________________________________. In many cases, when precision is high and accuracy is low, the fault can lie with the instrument. If a balance or a thermometer is not working correctly, they might consistently give inaccurate answers, resulting in high _________ and low ________. A dartboard analogy is often used to help students understand the difference between accuracy and precision. Imagine a person throwing darts, trying to hit the bull's-­‐eye. The closer the dart hits to the bull's-­‐eye, the more ______________ his or her tosses are. If the person misses the dartboard with every throw, but all of their shots land close together, they can still be very _________________. You must strive for both accuracy and precision in all of your laboratory activities this year. Make sure that you understand the workings of each instrument, take each measurement carefully, and recheck to make sure that you have ___________. Without accurate and precise measurement your calculations, even if done correctly, are quite useless. THE SHORT ANSWER: ACCURACY -­‐how close you are to an accepted value -­‐IN GRAPHS: how close your average (or sometimes slope) is to the true value PRECISION -­‐how close your measurements are to each other -­‐refers to the uncertainty in the measurement IN GRAPHS: how close all your data points are to the best fit line SEE p. 75 in Heath: Figure at the bottom