Chemical Bonding II: Molecular Geometry and Hybridization of
advertisement

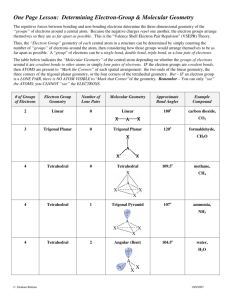
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Molecular Geometry • Problem: Lewis structures show connectivity between atoms, but not overall geometry of molecule • The geometry that the molecule ultimately assumes is determined by electron repulsion • Bonding and lone pair electrons are positioned as far apart from each other as possible • We need a model to describe the molecular geometry of molecules Molecular Geometry Valence shell electron pair repulsion (VSEPR) model: Predicts the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB2 2 0 linear linear B B 10.1 1 0 lone pairs on central atom Be Cl Cl 2 atoms bonded to central atom 10.1 Molecular Geometry VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 linear linear 0 trigonal planar trigonal planar AB3 3 Arrangement of electron pairs Molecular Geometry 10.1 Molecular Geometry BF3 10.1 2 Molecular Geometry VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 linear linear trigonal planar tetrahedral Arrangement of electron pairs AB3 3 0 trigonal planar AB4 4 0 tetrahedral Molecular Geometry 10.1 10.1 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 linear linear trigonal planar Arrangement of electron pairs Molecular Geometry AB3 3 0 trigonal planar AB4 4 0 tetrahedral tetrahedral 0 trigonal bipyramidal trigonal bipyramidal AB5 5 10.1 3 10.1 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 linear linear trigonal planar Arrangement of electron pairs Molecular Geometry AB3 3 0 trigonal planar AB4 4 0 tetrahedral tetrahedral trigonal bipyramidal octahedral AB5 5 0 trigonal bipyramidal AB6 6 0 octahedral 10.1 10.1 4 10.1 Molecular Geometry • 2 types of molecules • A molecule without lone pair of electrons on central atom • A molecule with lone pair of electrons on central atom • Lone pair of electrons are more repulsion, occupy more space, and contain more electron density than bonding electrons Lone pair e- CH4 bonding-pair vs. bonding-pair repulsion NH3 < lone-pair vs. bonding pair repulsion H 2O < lone-pair vs. lone-pair repulsion 5 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB3 3 0 AB2E 2 1 Arrangement of electron pairs Molecular Geometry trigonal planar trigonal planar trigonal planar bent 10.1 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB4 4 0 tetrahedral tetrahedral AB3E 3 1 tetrahedral trigonal pyramidal 10.1 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB4 4 0 tetrahedral tetrahedral AB3E 3 1 tetrahedral trigonal pyramidal AB2E2 2 2 tetrahedral bent O H H 10.1 6 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB5 5 AB4E 4 Arrangement of electron pairs Molecular Geometry 0 trigonal bipyramidal trigonal bipyramidal 1 trigonal bipyramidal distorted tetrahedron Seesaw 10.1 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB5 5 0 AB4E 4 1 AB3E2 3 2 Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron T-shaped F F Cl F 10.1 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB5 5 0 AB4E 4 1 AB3E2 3 2 AB2E3 2 3 Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron trigonal bipyramidal T-shaped linear I I I 10.1 7 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB6 6 0 octahedral octahedral AB5E 5 1 octahedral square pyramidal F F F Br F F 10.1 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB6 6 0 octahedral octahedral AB5E 5 1 octahedral AB4E2 4 2 octahedral square pyramidal square planar F F Xe F F 10.1 10.1 8 Predict the geometries of the following molecules and ion using the VSEPR method: (a) CBr4 (b) BCl3 (c) NF3 (d) H2Se (e) NO2(f) TeCl4 Geometry of Molecules with More Than One Central Atom • Draw Lewis structure of methanol • It’s difficult to determine overall shape of molecule, but we can determine shape around central atoms • Central atoms are C and O • What’s the geometric shape around C and O? Predicting Molecular Geometry 1. Draw Lewis structure for molecule. 2. Count number of lone pairs on the central atom and number of atoms bonded to the central atom. 3. Use VSEPR to predict the geometry of the molecule. 10.1 9 Dipole Moments • Dipole moments are used to determine whether or not a molecule is polar or non-polar overall • Why do you care? • Rule: Like dissolves like • Polar molecules are miscible in polar molecules (vice versa) Dipole Moments and Polar Molecules Shift in electron density (due electronegativity) can be quantitatively measured as a dipole moment (µ) m=Qxr electron poor region electron rich region H F δ+ δ- Q is the charge (C) r is the distance between charges (m) Polar molecule 1 D = 3.36 x 10-30 C m 10.2 Proof of polarity (partially + and – charges) is their alignment in electric field 10.2 10 Sum of all dipoles results in overall dipole of molecule, and determines polarity (polar or non-polar) 10.2 Which of the following molecules have a dipole moment? H2O, CO2, SO2, and CH4 Typically, symmetrical molecules are non-polar 10.2 Does BF3 have a dipole moment? No (non-polar molecule) 10.2 11 Does CH2Cl2 have a dipole moment? 10.2 There are three different structures of C2H2F2 are they all polar? Isomers Dipole Moments Other polar molecules: HCl, HF, H2O, SO2, and NO Non-polar molecules: H2, O2, F2, CO2, CH4, and benzene 10.2 12 Chemistry In Action: Microwave Ovens Dipole Moments at Work Microwaves are generated in magnetron Negative pole follows + region of wave Guided and reflected to food by waveguide and rotating blades, respectively Polar molecules mimic oscillating wave, therefore they rotate; collisions with food causes it to heat up 13