European Journal of Medicinal Chemistry 45 (2010) 1912–1918
Contents lists available at ScienceDirect
European Journal of Medicinal Chemistry
journal homepage: http://www.elsevier.com/locate/ejmech
Original article
Reaction mechanisms of allicin and allyl-mixed disulfides with proteins and small
thiol molecules
Talia Miron a, *, Irving Listowsky b, Meir Wilchek a
a
b
Department of Biological Chemistry, Weizmann Institute of Science, Rehovot 76100, Israel
Department of Biochemistry, Albert-Einstein College of Medicine, Bronx, NY, USA
a r t i c l e i n f o
a b s t r a c t
Article history:
Received 15 October 2009
Received in revised form
14 January 2010
Accepted 15 January 2010
Available online 21 January 2010
Allylsulfides from garlic are chemopreventive agents. Entering cells they are expected to initially interact
with glutathione. Accordingly, reaction mechanisms of the product, S-allylthio-glutathione, with model
proteins and thiols were analyzed in cell free systems. With glutathionyl, cysteinyl or captopril representing S-allyl aliphatic adducts, the reaction with sulfhydryl groups resulted in mixed disulfide
mixtures, formed by both, S-allyl and aliphatic moieties.
To improve conventional prodrug treatment of blood pressure, cancer and intestinal inflammation
S-allylthio prodrugs, such as S-allylthio-6-mercaptopurine and S-allylthio-captopril were synthesized.
Synergistic activities of the 2 constituents, as well as increased cell permeability allow for efficient in vivo
activity. Upon reaction of these derivatives with glutathione, S-allylthio-glutathione is formed, while
6-mercaptopurine is the leaving group. Excess cellular glutathione enables several cycles of sulfhydryldisulfide exchange reactions to occur, extending the hybrid drug’s pharmacodynamics.
Ó 2010 Elsevier Masson SAS. All rights reserved.
Keywords:
Allicin
Glutathione
S-Allylthio-mixed disulfide
Prodrug
Mechanism of action
1. Introduction
Allicin, diallyl thiosulfinate, is the major biologically active
compound derived from garlic. It is produced by the interaction of
the enzyme alliinase (alliin lyase; EC 4.4.1.4) with its substrate,
alliin (S-allyl-L-cysteine sulfoxide) (Scheme 1) [1].
Allicin is a short-lived compound which easily diffuses through
cell membranes (diffusion coefficient 5 108 cm2 s1) [2] and
exerts its biological effects by rapidly reacting with intracellular
free thiols, such as reduced glutathione (GSH), cysteine and sulfhydryl groups of proteins. The reaction of the allylthio group with
those cellular components constitutes the major beneficial effects
of allicin. The first product is most likely that of the S-allylthiomixed disulfide (AS-SX) with GSH as depicted in Scheme 2 below.
Intracellular GSH is the major low molecular weight thiol that
is present at millimolar concentrations in many cell types [3,4]
Abbreviations: ASH, allylmercaptan; AS-SX, S-allylthio-mixed disulfide; CPSH,
captopril; CPSSA, S-allylthio-captopril; DTNB, 5,50 -dithio-bis (2-nitrobenzoic acid);
G3PDH, glyceraldehyde 3-phosphate dehydrogenase GSSA, S-allylthio-glutathione;
GSH, Reduced glutathione; GSSG, Glutathione oxidized; GS-S-CP, S-glutathionylthiocaptopril: NTB, 2-nitro-5-thiobenzoate; PTP1B, Protein tyrosine phosphatase
1B; SA-6MP, S-allylthio-6-mercaptopurine; SA-6MPR, S-allylthio-6-mercaptopurine
riboside.
* Corresponding author. Tel.: þ972 8 9343627; fax: þ972 8 9468256.
E-mail addresses: talia.miron@weizmann.ac.il (T. Miron), irving@medusa.bioc.
aecom.yu.edu (I. Listowsky), meir.wilchek@weizmann.ac.il (M. Wilchek).
0223-5234/$ – see front matter Ó 2010 Elsevier Masson SAS. All rights reserved.
doi:10.1016/j.ejmech.2010.01.031
and participates in many important biological processes,
including maintenance of a reducing intracellular environment
[5] and detoxification of oxidants and electrophiles [6]. GSH also
participates in cellular redox reactions and mixed disulfide
formation, which leads to the production of S-glutathiolated
proteins [7–10].
The mechanisms by which the allylsulfides reduce the risk of
diseases may be rationalized on the basis of their chemistry [11].
Thus, they could affect GSH levels and cellular redox status, or
react directly with key proteins involved in various physiological
processes. However, details of the functional course of action of
allylsulfides are obscure. Since the initial cellular products are
likely to be GSH adducts, this study was designed to determine
the outcome and reaction mechanisms of S-allylthio-glutathione
(GSSA) with model proteins and low molecular weight thiols.
The putative products, the mixed disulfide, can in turn, be
involved in further exchange reactions with free thiols,
potentially modulating various physiological processes in the cell
[12–14].
Several S-allylthio-mixed disulfide compounds (AS-SX) were
prepared (including drugs containing free thiol groups) and their
disulfide exchange reactions with GSH and proteins containing free
sulfhydryl groups were studied, in order to follow the formation of
the various intermediates and final products. These substances
could shed light on the reaction mechanisms of S-allylthio-mixed
disulfide (AS-SX) with cellular thiols.
T. Miron et al. / European Journal of Medicinal Chemistry 45 (2010) 1912–1918
1913
Scheme 1. Enzymatic production of allicin.
2. Chemistry
All of the S-allylthio-derivatives were synthesized by coupling
allicin to the indicated sulfhydryl-derivatives at pH 6.5, using the
ratio of allicin: SH-derivatives 1:1.8 (Scheme 3).
3. Results
3.1. Reactions of S-allylthio-glutathione (GSSA) with sulfhydryl
groups of proteins
Papain was incubated with GSSA to identify mixed disulfide
derivatives formed during the reaction. Previously we showed that
modification of papain with GSSA abolishes its enzymatic activity
in a concentration dependent manner [15]. GSSA modification of
papain in this study caused a decrease in catalytic activity in
a biphasic manner, reaching a total loss of activity after 18 h. These
results are consistent with the formation of a papain-S-SX derivative. Using the tritiated allyl moiety ([3H]GSSA) enabled tracking
the formation of modified papain products. Gel permeation chromatography of the reaction mixture using a PD-10 column, suggested that a major product is [3H] allyl-S-S papain since the
radioactivity and protein peaks overlap (Fig. 1). In the 4th–5th ml
peak, the ratio is 0.7–0.9 mCi labeled allyl group per mmole protein,
implying that at least 70% of the protein is S-allylthio-papain.
However, to determine whether other derivatives, such as
glutathionyl-S-S-papain were also formed papain was modified
with unlabeled GSSA, and protein mixed disulfide formation was
analyzed by ESI-MS. Unmodified papain showed the presence of
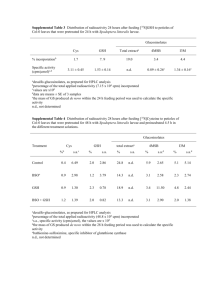
two major species [16] with molecular masses of 23 428 Da (a) and
23 458 Da (b). Modified papain revealed the formation of 2 distinct
products derived from each respective form. Since papain has only
one free sulfhydryl group, the modification results in the formation
of either S-allylthio-papain (additional mass of 73 Da, with or
without Naþ) or S-glutathionyl papain (additional mass of 306 Da,
with or without Naþ) (Table 1).
Papain was also reacted with S-allylthio-captopril (CPSSA). Mass
spectra of the products indicated that two distinct modifications
had occurred; S-captropril-papain (23 643 kDa, additional mass of
215 Da) and S-allylthio-papain (23 506, 23 531 kDa, additional mass
of 73 Da).
Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) is
a protein containing a reactive sulfhydryl group and known to
undergo S-glutathionylation under conditions of oxidative stress
[17]. After incubation of the enzyme with a ten-fold molar excess of
GSSA at pH 7.4, the products were analyzed by HPLC and ESI-MS.
The presence of roughly equivalent amounts of unmodified G3PDH
(35 778 Da) as well as modified S-glutathionylated G3PDH
(36 084 Da) were obtained without substantial loss of enzymatic
Scheme 2. The reaction of allicin with GSH.
activity. The maximum extent of S-glutathionylation of the nondenatured enzyme under these conditions is 2-per tetramer. PTP1B
has 6 cysteine residues including a very reactive sulfhydryl at the
active site. Two major reaction products were obtained in the
reaction of PTP1B and GSSA. These included a 37 453 Da component
indicating the addition of 2 allylmercapto moieties, and a 37 758 Da
component indicative of the addition of 2 allylmercapto moieties as
well as a single S-glutathionylated group linked to the protein.
3.2. Reaction between allicin and GSH
All of the S-allylthio-derivatives were prepared using an excess
of allicin. The reaction was carried out at room temperature, pH 6.5.
Under these conditions the product is stable and can be readily
isolated. When allicin (5 mM) reacted with excess of GSH (50 mM),
the first product formed rapidly was GSSA, which ultimately was
converted back to GSH, while releasing allylmercaptan (ASH). Only
traces of ASH were detected by HPLC (due to its high volatility).
3.3. Reaction between S-allylthio-captopril (CPSSA) and GSH
CPSSA [18] and GSH were reacted at a molar ratio of 1:1. The
data in Table 2A show molecular masses of the various reactants,
intermediates and products, as established by ESI-MS and HPLC
retention times (Rt).
The time course of product formation in the reaction mixture of
S-allylthio-captopril (CPSSA) and GSH at room temperature, pH 6.5
is shown in Table 2B.
ESI-MS analysis of the reaction mixture HPLC peaks indicated
that it contained S-glutathionyl-captopril (GS-S-CP: mw 522, Rt:
4.6 min), S-allylthio-glutathione (GSSA: mw 379, Rt: 5.2 min) free
captopril: (CPSH: mw 217, Rt:13.0 min) as well as the starting
materials (Table 2B). The products that appear initially are GSSA
and CPSH. At a later stage GS-S-CP, GSSG and allylmercaptan (ASH)
are formed. In this reaction, reduced glutathione reacted with both
moieties of S-allylthio-captopril to yield at first, S-allylthio-glutathione (GSSA) and captopril (CPSH). Only at later stages do these
intermediates react with one another to yield the final product,
S-glutathionyl-captopril (GS-S-CP).
The reaction is pH dependent and at pH 8.4 the maximal yield of
GS-S-CP, GSSA and CPSH were observed after 25 min at room
temperature. To obtain more precise values of reaction rates and
intermediate analyses, the reaction was performed at pH 6.5. The
overall reaction is shown in Scheme 4.
The second step of the above reaction was deduced from the
kinetics of product formation at pH 6.5 as shown in Fig. 2.
As CPSH reacted with GSSA (at equimolar ratios of approximately 9 mM, pH 6.5 at room temperature), the products GSH and
GS-S-CP formed after 2 min and their amounts increased for
80 min, attaining steady state levels for up to 20 h. About 60% of the
starting material (GSSA) was converted to GSH after 80 min, which
indicates that S-glutathionyl is the preferred leaving group. The
formation of CPSSA was observed after 20 min and increased with
time, reaching its maximal amount after 2.5 h. After 20 h, CPSH and
GSSA (the starting materials) could not be detected and the reaction mixture contained GSH (6.3 mM), GS-S-CP (2.8 mM) and CPSSA
1914
T. Miron et al. / European Journal of Medicinal Chemistry 45 (2010) 1912–1918
Scheme 3. General synthesis of S-allylthio-derivatives.
(4.1 mM), that is 70%, 31% and 46% from each of the starting reactants, respectively (Fig. 2). The oxidized forms, GSSG and captopril
disulfide (CPSSCP) were also observed in the reaction mixture at
room temperature after 20 h at pH 8.4.
3.4. Reaction of S-allylthio-6-mercaptopurine riboside (SA-6MPR)
and S-allylthio-6-mercaptopurine with GSH
GSH and SA-6MPR [19]were mixed at equimolar concentrations
(7 mM, in 50 mM phosphate buffer, pH 7.2 at room temperature).
The reaction was very rapid and after 10 min, SA-6MPR disappeared. The products were identified by HPLC and ESI-MS analysis. Optical spectra of the reaction mixture showed a shift of the
absorption maximum from 284 nm (SA-6MPR) to 324 nm (6-MPR)
(Fig. 3). In order to decrease the reaction rates, the reaction was
performed at pH 6.0. Samples from the reaction mixture were taken
at different time points, diluted with 50% ethanol and spectra were
measured.
After 30 min, a peak of absorbance at 324 nm indicated the
presence of 6-MPR, whereas no SA-6MPR could be detected. The
isosbestic points revealed the presence of two distinct compounds
in solution without any indication of intermediate products.
Further evidence was obtained from ESI-MS analysis, which indicated the presence of only 6-MPR and GSSA.
SA-6MP [19] reacted with GSH in a similar manner, the only
products being GSSA and 6-MP, but this reaction occurred at
a faster rate. In both cases the leaving groups are 6-MPR or 6-MP
and the mixed disulfide formed is GSSA. Scheme 5 describes the
products formed by the reaction of SA-6MP with GSH.
3.5. Reaction of S-allylthio-6-mercaptopurine (SA-6MP) and 2nitro-5-thiobenzoate (NTB)
The high activity of SA-6MPR and SA-6MP was also demonstrated by their reaction with 2-nitro-5-thiobenzoate (NTB) [20]. In
this case the mercaptopurine is the leaving group and S-allylthioNTB is formed (Scheme 6).
Based on the absorbance decrease of NTB in this reaction, we
developed a spectrophotometric assay to determine the concentration of SA-6MP and SA-6MPR (e412 14 150 M1 cm1) (unpublished data).
4. Discussion
Allicin reacts with free thiol groups of proteins and GSH. During
uptake by cells some of it reacts with thiol containing membrane
proteins [21] but the major product is GSSA. The life span of allicin
in cells is short due to its volatility and instability. To bypass this
problem we devised cell penetrable S-allylthio disulfide-derivatives
Table 1
ESI-MS data of papain modified with GSSA and with CPSSA.
Papain
Papain (SH)a
Papain (SH)b
Fig. 1. Chromatographic pattern of Radioactivity (C) and protein concentration (B) of
modified papain. Chromatography was carried out on PD-10 column equilibrated with
50 mM Na acetate, 2 mM EDTA pH 6.2. Fractions volume 0.8 mL.
Delta MW
Exp MW
0
0
MS observed
23 428
23 458
Papain/GSSA
S-allylthio-papain (a)
S-allylthio-papain (b)
S-allylthio-papain (b)/Naþ
S-glutathionyl papain (b)
S-glutathionyl papain (b)/Naþ
73
73
96
305
328
23
23
23
23
23
Papain/CPSSA
S-allylthio-papain (a)
S-allylthio-papain (b)
S-captopril-papain (a)
73
73
215
23 501
23 531
23 643
501
531
554
763
786
23
23
23
23
23
506
531
554
763
789
23 506
23 531
23 643
T. Miron et al. / European Journal of Medicinal Chemistry 45 (2010) 1912–1918
Table 2A
Mass and retention time of various glutathionyl and captopril derivatives.
Compound
GSSG
GSH
GS-S-CP
GSSA
CPSH
CPSSA
MS (Da)
HPLC Rt (min)
612
4.1
307
4.3
522
4.6
379
5.2
217
5.8
289
13.0
Table 2B
Time-scale evolution of products in the reaction of GSH with CPSSA (mM).
Reaction
time (h)
0
0.1
0.3
0.6
1.0
3.0
20.0
GSSG
GSH
0.3 0.1
10.0 0.3
9.3 0.3
7.5 0.4
7.1 0.2
7.0 0.2
6.8 0.2
6.3 0.2
GS-S-CP
GSSA
1.5
2.0
3.5
3.5
0.5
1.2
1.1
2.3
2.5
2.1
0.3
0.3
0.2
0.2
CPSH
0.1
0.1
0.1
0.1
0.1
0.2
0.4
0.9
1.3
1.9
2.3
2.1
CPSSA
0.2
0.1
0.1
0.1
0.2
0.1
9.5
9.0
7.2
6.7
5.5
3.1
2.8
0.4
0.4
0.1
0.1
0.2
0.1
0.1
Results represent means S.D. of three independent experiments.
(AS-SX) to react with GSH via sulfhydryl-disulfide exchange reactions. Several mercapto containing prodrugs were employed to
prepare AS-SX derivatives and their interaction with GSH and
proteins was analyzed in cell free systems. Attempts were made to
improve the pharmacological performance of the prodrugs and to
identify GSSA reaction products, thus enabling the evaluation of
their potential as new hybrid prodrugs. The poor cell penetrability
of the original drugs was overcome by the addition of a hydrophobic allylmercapto group. Upon reaction with excess intracellular
GSH the desired intracellular GSSA would be formed, while
releasing the original prodrug inside the cells, thus obtaining
a synergistic effect of the original prodrug and a series of beneficial
redox reactions.
Reactions between S-allylthio-mixed disulfides and free sulfhydryl groups are non-enzymatic thiol-disulfide exchange reactions. They usually occur spontaneously at pH > 5.5, reactivity
1915
increasing with the increase in the basicity and the nucleophilic
capacity of the thiolate anion. The nature of the leaving thio moiety
depends on the substituent involved.
Analysis of the reactions between S-allylthio-derivatives with
either reduced glutathione or with model proteins containing
a reactive sulfhydryl group provided information on the process of
intermediate and final product formation.
The S-allylthio-mixed disulfides used contained an S-allyl group
and one S-aliphatic or S-aromatic moiety. In the case of glutathionyl, cysteinyl or captopril as the S-aliphatic moieties, the
reaction with sulfhydryl groups of proteins resulted in mixed
disulfide mixtures that are formed by both the S-allyl as well as by
the aliphatic moieties, as exemplified by the reaction of GSSA with
papain and PTP1B. The reaction between GSSA and papain yielded
S-allylthio-papain and Glutathionyl-S-S-papain. The modification
(loss of enzyme activity) takes several hours. Both products, ASSprotein and GSS-protein are stable in a cell free system, whereas in
cells the reaction is probably reversible due to intervention of
various enzymes such as thioredoxins and glutaredoxins [22].
The S-allyl mixed disulfide exchange reactions may be represented by Scheme 7.
When S-allyl mercaptocaptopril (CPSSA) reacted with reduced
glutathione (GSH) (equimolar concentrations), all the possible
product combinations (GSSA, CPSH and GS-S-CP) were detected.
The early appearance of GSSA and CPSH indicated that captopril in
the mixed disulfide is a better leaving group than the S-allylthio
moiety. Upon longer incubation, GS-S-CP appeared in the reaction
mixture, reaching its maximal level at 3 h, and staying at that level
for up to 20 h. It was also found that GSSA and CPSH, both formed in
this reaction, proceed to react with, each other, to yield GS-S-CP,
which is a stable mixed disulfide in the cell free system. While stage
1 of the reaction (Scheme 8) showed full mass conservation of the
glutathione and the allyl moieties, the recovery of the S-allylthio
moiety at stage 2 was only 50%. The explanation for this loss was
deduced from the strong odor emitted by allylmercaptan, a volatile
compound formed by this leaving group.
Scheme 4. The reaction of GSH with CPSSA.
1916
T. Miron et al. / European Journal of Medicinal Chemistry 45 (2010) 1912–1918
Fig. 2. Kinetics of product formation in the reaction mixture of GSSA and CPSH at ambient temperature pH 6.5. A. The reactant GSSA (B) and the reaction products GSH (C) and GSS-CP (-). B. The starting reactant captopril (CPSH (B)) and the reaction products of GS-S-CP (-) and CPSSA (C).
The 2 step reaction of GSH with CPSSA is presented in Scheme 8.
Thus, the aliphatic moiety of S-allyl mixed disulfides generates
various mixed disulfides in the thiol-disulfide exchange reactions,
whereas the aromatic moiety does not (Scheme 7). In the reactions
of SA-6MP or SA-6MPR, with an aliphatic free SH such as GSH,
a very fast release of 6-MP or 6-MPR occurred. The same situation
applies to the free thiol of the aromatic NTB. 6-MPR and 6MP are
not sufficiently nucleophilic to react with GSSA.
SA-6MP and SA-6MPR are potential anti-cancer prodrugs. The
mode of action of these compounds with free sulfhydryl groups
suggests that these promising lipophilic prodrugs, upon entering
the living cells will promptly react with GSH and release the purine
moiety inside the cells, where it can act as a purine analogue and
interfere with DNA synthesis. The allyl moiety contributes to the
lipophilicity of these compounds, and hence to their increased
capacity for cell membrane penetration. The S-allyl modified
disulfide activity lasts longer as compared to the parent molecule
(allicin). While this study was performed at molar 1:1 ratios of
reactants in order to determine all the intermediates and products,
the amount of GSH in cells is in large excess (mM range) in relation
to allicin or S-allylmercapto-drugs (mM or nM).
Furthermore, the S-allyl moiety will form intracellular GSSA that
may continue to modify reactive sulfhydryl groups, yielding various
products, either small or high-molecular weight mixed disulfides,
further subjected to mixed disulfide exchange reactions. Additionally, the allylmercaptan (ASH) moiety reacts with metalloproteins. In
the case of histone deacetylase (HDAC), for instance, inhibition of
activity was observed due to its binding to zinc in the active site [23].
The rest of the allylmercaptan is released by evaporation (Scheme 9).
The fact that allicin has never been detected in mammalian
blood, urine or stool even following a short period after administering/consuming large amounts of the purified compound or garlic
in its raw form [24,25] can be explained by its immediate conversion into the above described cellular mixed disulfides, GSSA,
Protein-S-SA or Protein-S-SG. However, the discovery of active
intermediates, even 20 h after reacting allyl derivatives with free
thiol bearing molecules, point out the promising prolonged activity
of the hybrid S-allylthio prodrugs in vivo. Not only has the time
scale, and the membrane penetrability improved significantly, but
the multiple intermediates formed suggest a variety of cellular
targets to be affected by these prodrugs.
5. Experimental
5.1. General
Fig. 3. Optical spectra of the reaction mixture of GSH and SA-6MPR at pH 6.0. Spectra
were measured at time 0 min (a); 10 min (b); 15 min (c) and 30 min (d).
Papain (EC 3.4.22.2) was obtained from Worthington (Freehold,
NJ). Protein tyrosine phosphatase 1B (PTP1B, (residues 1–321) EC
3.1.3.48) was a gift from Dr. Zhong-Yin Zhang [26]. Rabbit muscle
glyceraldehyde 3-phosphate dehydrogenase (G3PDH, EC 1.2.1.12),
5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB, Ellman’s Reagent),
6-mercaptopurine (6-MP), captopril, L-cysteine and reduced
glutathione (GSH) were purchased from Sigma (St. Louis, MO).
Porapak Q (100–120 mesh) was obtained from Waters Associates,
(Milford, MA, USA); PD-10 (pre-packed Sephadex G-25) from
Pharmacia LKB, Biotechnology, Uppsala, Sweden. Allicin was
produced as previously described [27]. Free sulfhydryl groups were
determined with DTNB [28] by using EM 14 150 M1 cm1 at 412 nm
according to Riddles et al. [29].
T. Miron et al. / European Journal of Medicinal Chemistry 45 (2010) 1912–1918
1917
Scheme 5. The reaction of S-allylthio-6-mercaptopurine (SA-6MP) with GSH.
Scheme 6. The reaction of SA-6MP with 2-nitro-5-thiobenzoate (NTB).
Modified papain was subjected to chromatography on a PD-10
column. HPLC fractionation of PTP1B and glyceraldehyde 3-phosphate dehydrogenase was done on Vydac C4 reversed-phase
column (1 250 mm). Protein peaks were eluted using increasing
linear gradients of acetonitrile/0.1% TFA (solvent B) at a flow rate of
0.05 mL/min, and were subjected to ESI-MS analysis.
HPLC analyses of low molecular weight compounds were performed on a LiChrosorb RP-18 (7 250 mm) column, using
methanol (60%) in water containing 0.01% trifluoroacetic acid, at
a flow rate of 0.55 mL/min, and absorbance was recorded at 210 nm.
1
HNMR spectra were measured on a Bruker Avance-500 spectrometer (Bruker, Bremen, Germany). Mass spectra of proteins were
analyzed by using ion electrospray ESI-MS. MALDI-TOF data were
collected on a Bruker Reflex IIIÔ MALDI-TOF mass spectrometer
(Bruker, Bremen, Germany) equipped with a delayed extraction ion
source, a reflector and a 337 nm nitrogen laser, and on an API
Q-STAR Pulsari Electrospray-Quadrupole TOF tandem mass spectrometer (MDS-Sciex, Canada, ABI) equipped with a nanoelectrospray source (MDS Proteomics, Odense, Denmark).
Mass spectra of small molecules were analyzed by using
Micromass Platform LCZ 4000, Micromass, Manchester, UK.
Ionization Mode: ESI-ElectroSpray ionization.
5.2. General synthesis procedures
Sulfhydryl-compounds (1 mmol) were added to an allicin solution (0.55 mmol, in phosphate buffer, pH 6.5). The reaction was
carried out at room temperature in 50% ethanol for several hours.
Excess allicin was removed by ether extraction. After solvent removal
under reduced pressure, the product was isolated from water.
5.2.1. Allicin [27](compound 1)
1
H NMR (400 MHz, D2O) d in ppm: 6.07 (m, 1H), 6.03 (m, 1H),
5.55 (dq, 2H), 5.37 (dq, 2H), 3.97 (dq, 2H), 3.85 (m, 2H).
5.2.2. S-allylthio-glutathione (GSSA) [2,15] (compound 2)
1
H NMR (400 MHz, D2O) d in ppm: 5.88 (m, 2H), 5.88 (m, 1H), 5.2
(m, 2H), 4.72 (dd. 1H), 3.76 (d, 2H), 3.74 (t, 1H), 3.36 (dd, 2H), 2.52
(q, 2H), 2.14 (q, 2H). MS (EI): [M þ 1] at m/z 380.
5.2.3. S-allylthio-captopril (CPSSA)[18] (compound 3)
1
H NMR (300 MHz, CDCL3) d in ppm: 5.80 (m, 1H), 5.15 (m, 2H),
4.55 (m, 1H), 3.64 (t, 2H), 3.29 (d, 2H), 3.03 (m, 2H), 2.64 (m, 1H),
2.44, 2.20 (m, 4H), 1.18 (d, 3H). MS (EI): [M þ 1] at m/z 290.
5.2.4. S-allylthio-6-mercaptopurine riboside (SA-6MPR) [19]
(compound 4)
1
H NMR (500 MHz, CDCL3) d in ppm: 8.87 (s, 1H), 8.10 (s, 1H),
5.88 (d, 1H) 5.87 (m, 1H), 5.12 (m, 2H), 5.12 (m, 1H), 4.55 (d, 1H),
4.39 (s, 1H), 390, (dd, 2H) 3.56 (d, 2H), MS (EI): [M þ 1] at m/z 357.
Scheme 7. Hypothetical products of the reaction between S-allylthio-mixed disulfide
and free SH compounds.
5.2.5. S-allylthio-6-mercaptopurine (SA-6MP) [19](compound 5)
1
H NMR (500 MHz, CDCL3) d in ppm: 12.19 (s, 1H), 8.96 (s, 1H),
8.30 (s,1H), 5.91 (m,1H), 5.14 (m, 2H), 3.61 (d, 2H). MS (EI): [M þ 1]
at m/z 225.
1918
T. Miron et al. / European Journal of Medicinal Chemistry 45 (2010) 1912–1918
Scheme 8. The S-allyl mixed disulfide exchange reactions (a 2 step general scheme).
quantitatively by HPLC separation at different time periods and
their molecular weight was determined by (ESI-MS).
Acknowledgement
This work was supported by grants from La Foundation Raphael
et Regina Levy and the Atran Foundation.
References
Scheme 9. A general scheme depicting the fate of allicin and S-allylmercapto derivatives in cells.
5.2.6. S-allylthio-2-nitro-5-thiobenzoate (SA-NTB) [20] (compound 6)
1
H NMR (400 MHz, DMSO) d in ppm: 8.0 (d, 1H), 7.86 (d, 1H), 7.84
(dd, 1H), 5.77 (m, 1H), 5.1 (m, 2H), 3.49 (d, 2H). [M þ Na] at m/z 294.
5.3. Modification of proteins with S-allyl derivatives
Protein modification was carried out at a ratio of protein: S-allyl
derivative (1:10) at pH 6.2–7.4 in buffer (acetate or phosphate) for
18–24 h. After purification proteins were subjected to ESI-MS
analysis.
5.4. The reaction of glutathione with various S-allylthio-derivatives
GSH was added at 1:1 or 10:1 M ratios to S-allyl mixed disulfide
(AS-SX), X representing; captopril, 6-mercaptopurine (6-MP) or 6mercaptopurine riboside (6-MPR), in phosphate buffer pH 5.5–7.2
at room temperature. The reaction products were analyzed
[1] A. Stoll, E. Seebeck, Adv. Enzymol. 11 (1951) 377–400.
[2] T. Miron, A. Rabinkov, D. Mirelman, M. Wilchek, L. Weiner, Biochim. Biophys.
Acta 1463 (2000) 20–30.
[3] H.J. Bremer, M. Duran, J.P. Kamerling, H. Przyrembel, S.K. Wadman, in:
H.J. Bremer, M. Duran, J.P. Kamerling, H. Przyrembel, S.K. Wadman (Eds.),
Disturbance of Amino Acid Metabolism: Clinical Chemistry and Diagnosis,
Urban & Schwarzenberg, Baltimore-Munich, 1981, pp. 80–82.
[4] A. Meister, M.E. Anderson, Annu. Rev. Biochem. 52 (1983) 711–760.
[5] F.Q. Schafer, G.R. Buettner, Free Radic. Biol. Med. 30 (2001) 1191–1212.
[6] D.F. Dourado, P.A. Fernandes, M.J. Ramos, Curr. Protein Pept. Sci. 9 (2008) 325–337.
[7] M.M. Gallogly, J.J. Mieyal, Curr. Opin. Pharmacol. 7 (2007) 381–391.
[8] D.M. Townsend, Mol. Interv. 7 (2007) 313–324.
[9] I. Dalle–Donne, A. Milzani, N. Gagliano, R. Colombo, D. Giustarini, R. Rossi,
Antioxid. Redox Signal. 10 (2008) 445–473.
[10] J.J. Mieyal, M.M. Gallogly, S. Qanungo, E.A. Sabens, M.D. Shelton, Antioxid.
Redox Signal. 10 (2008) 1941–1988.
[11] J. Milner, J. Nutr. 136 (2006) 827S–831S.
[12] J.T. Pinto, C. Qiao, J. Xing, R.S. Rivlin, M.L. Protomastro, M.L. Weissler, et al., Am.
J. Clin. Nutr. 66 (1997) 398–405.
[13] S. Biswas, A.S. Chida, I. Rahman, Biochem. Pharmacol. 71 (2006) 551–564.
[14] S.K. Jensen, R.E. Hansen, J.R. Winther, Antioxid. Redox Signal.11 (2009) 1047–1058.
[15] A. Rabinkov, T. Miron, D. Mirelman, M. Wilchek, S. Glozman, E. Yavin, et al.,
Biochim. Biophys. Acta 1499 (2000) 144–153.
[16] M. Azarkan, A. El Moussaoui, D. van Wuytswinkel, G. Dehon, Y. Looze,
J. Chromatogr. B 790 (2003) 229–238.
[17] I.A. Cotgreave, R. Gerdes, I. Schuppe-Koistinen, C. Lind, Meth. Enzymol. 348
(2002) 175–182.
[18] T. Miron, A. Rabinkov, E. Peleg, T. Rosenthal, D. Mirelman, M. Wilchek, Am. J.
Hypertens. 17 (2004) 71–73.
[19] T. Miron, F. Arditti, L. Konstantinovski, A. Rabinkov, D. Mirelman, A. Berrebi, et
al., Eur. J. Med. Chem. 44 (2009) 541–550.
[20] T. Miron, A. Rabinkov, D. Mirelman, L. Weiner, M. Wilchek, Anal. Biochem. 265
(1998) 317–325.
[21] M. Patya, M.A. Zahalka, A. Vanichkin, A. Rabinkov, T. Miron, D. Mirelman, et al.,
Int. Immunol. 16 (2004) 275–281.
[22] Y. Meyer, B.B. Buchanan, F. Vignols, J.P. Reichheld [Epub ahead of print]. Annu.
Rev. Genet. (2009).
[23] H. Nian, B. Delage, J.T. Pinto, R.H. Dashwood, Carcinogenesis 29 (2008) 1816–1824.
[24] L.D. Lawson, Z.J. Wang, Planta Med. 59 (Suppl.) (1993) A688–A689.
[25] F. Freeman, Y. Kodera, J. Agric. Food Chem. 43 (1995) 2332–2338.
[26] Y.L. Zhang, Z.Y. Zhang, Anal. Biochem. 261 (1998) 139–148.
[27] T. Miron, H. SivaRaman, A. Rabinkov, D. Mirelman, M. Wilchek, Anal. Biochem.
351 (2006) 152–154.
[28] G.L. Ellman, Arch. Biochem. Biophys. 82 (1959) 70–77.
[29] P.W. Riddles, R.L. Blakeley, B. Zerner, Anal. Biochem. 94 (1979) 75–81.