Test #1 Study Guide 1. Review basic lab safety rules. 2. Review
advertisement

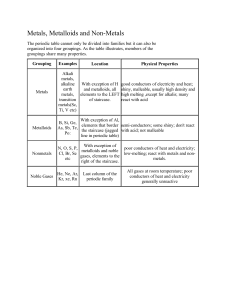
Test #1 Study Guide 1. Review basic lab safety rules. 2. Review laboratory equipment worksheet. 3. Be sure to know all element flashcards. 4. Chemistry is the study of the __composition____________ , __structure_______________, and __properties_________________ of matter. 5. What are the six branches of chemistry? _Organic, Inorganic, Physical, Analytical, Biochemistry, and Theoretical_________________________________________________________________ 6. What is studied in each of these branches? (Review Chemistry is a Physical Science questions) 7. Basic research is performed to __gain knowledge____________________. 8. Applied research is performed to ___solve problems___________________________. 9. Technology is the _application________ of __knowledge___________ to make life easier. 10. Anything that has takes up space and has mass is known as _matter________________. 11. Matter is classified into _substances_______ and ____mixtures___________________. 12. Name the two kinds of substances. compound____________ and ___element___________ 13. Name the two types of mixtures. _heterogeneous________ and ____homogeneous________ 14. How are the two types of mixtures distinguished? Homogeneous - uniform_____________heterogeneous - nonuniform________________ 15. A compound is made up of two or more elements that are chemically _bonded____________ to one another. 16. A substance made up of only one kind of atom is a(n) _element_________________. 17. What type of substance can be separated by ordinary chemical means? _compound________ 18. What is the definition of a mixture? Blend of two or more kinds of matter each retaining its own identity_______________________________________________ 19. A _chemical________________ change produces new substances with new and different physical properties. 20. After a __physical________________ change, the material is the same chemical make-up as it was before the change. 21. Classify the following changes as chemical or physical. __C___ silver tarnishing __P__ paper shredding in a shredder __C___ iron rusting __P___ butter melting in a dish __P___ sublimation of frozen water in a freezer __P___ sugar dissolving in tea __C___ apple juice fermenting __C___ bread baking __C___ baking soda and vinegar reacting __C___ log burning __C___ food digestion __P___ steel melting __P___ ice cream freezing in ice cream churn __P___ distillation of alcohol 22. Classify the following as physical properties or chemical properties. __C___ metal reacting in acid to produce hydrogen gas __P___ solubility __P___ color __C___ burning __P___ taste __C___ reacts with water __P___ odor __C___ reacts with base __P___ density __P___ boiling point __C___ explosive __P___ melting point _C____ flammable _C____ neutralizes acids _P____ refractive index _P____ luster 23. Elements are classified as _metals_______________, __metalloids_______________, and __nonmetals________________________. 24. What type of element is the greatest in number? __metals_________________ 25. The _metalloids___________________ are found along the stair – step line. They are sometimes referred to as __semiconductors___________________ of electricity. 26. Classify the following characteristics as those of metals (M), nonmetals (NM) or metalloids (S). __M___ good conductor of electricity __M___ malleable __M___ good conductor of heat __M___ ductile __NM___ dull and brittle __M___ shiny __S___ semiconductors _NM____ most are gases __M___ most are solids __NM___ poor conductor of electricity 27. All of the elements are organized into a table called the _periodic_________________ table. The horizontal rows are called ___periods_________________. The vertical columns are called __groups______________. 28. All of the elements have their own chemical _symbol______________ which is usually the first letter or first and second letter of their name. 29. What are the four states of matter? _plasma________________, _solid______________, _liquid______________, __gas_____________ 30. Which state of matter has a definite shape and definite volume? _solid_____________ 31. Which state of matter has a definite volume but not a definite shape? _liquid___________ 32. Which state of matter does not have a definite volume or shape? _gas_____________ 33. Which state of matter is not made up of atoms but of charged particles? _plasma____________ 34. Which group of the periodic table is called the noble gases? _18___________What is special about the noble gases? ___unreactive___________________________________________ 35. Where are the metals located on the periodic table? _left of staircase__________________ Where are the nonmetals located? _right of staircase____________________ 36. What is the name for group 1? Alkali metals_______ Group 2? Alkaline earth metals_____ Groups 3 through 12? Transition metals__________ Group 17? ___halogens________ 37. A chemical change is represented with a chemical equation_________________. The reactants____________ are on the left side of the arrow and the _products_______________ are on the right side of the arrow. 38. What is the difference between a chemical property and a physical property? _chemical – ability to undergo change into new substances____________________________ 39. Extensive properties depend on the amount of matter and intensive properties are independent of the amount of matter present. Classify the following as extensive or intensive physical properties. _E____ mass _I____ boiling _I____ color _E____ height _E____ length _I____ odor _E____ solubility _I____ density _E____ volume __I___ reactivity