Physical and Chemical Properties
advertisement
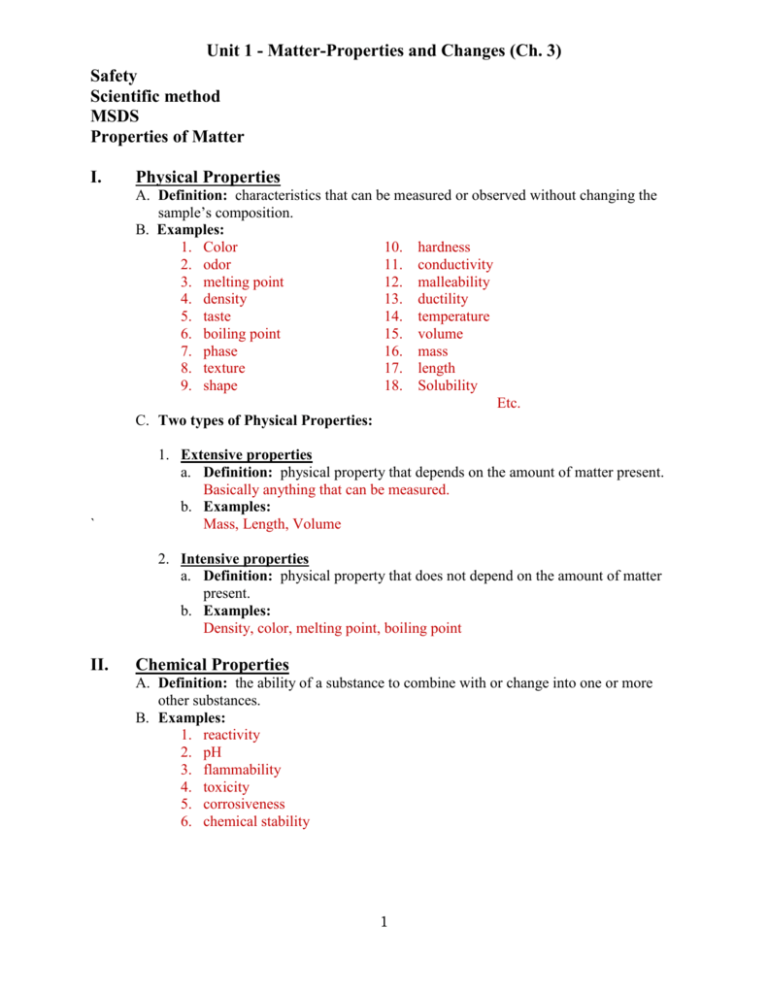
Unit 1 - Matter-Properties and Changes (Ch. 3) Safety Scientific method MSDS Properties of Matter I. Physical Properties A. Definition: characteristics that can be measured or observed without changing the sample’s composition. B. Examples: 1. Color 10. hardness 2. odor 11. conductivity 3. melting point 12. malleability 4. density 13. ductility 5. taste 14. temperature 6. boiling point 15. volume 7. phase 16. mass 8. texture 17. length 9. shape 18. Solubility Etc. C. Two types of Physical Properties: ` 1. Extensive properties a. Definition: physical property that depends on the amount of matter present. Basically anything that can be measured. b. Examples: Mass, Length, Volume 2. Intensive properties a. Definition: physical property that does not depend on the amount of matter present. b. Examples: Density, color, melting point, boiling point II. Chemical Properties A. Definition: the ability of a substance to combine with or change into one or more other substances. B. Examples: 1. reactivity 2. pH 3. flammability 4. toxicity 5. corrosiveness 6. chemical stability 1 III. States of Matter Solid has definite shape and definite volume On the periodic table, solids at room temperature are denoted by black Liquid flows, has definite volume but no definite shape On the periodic table, liquids at room temperature are denoted by blue Gas - has no definite shape and no definite volume The term gas refers to a substance that is naturally in the gaseous state at room temperature. On the periodic table, gases are denoted by red. The term vapor refers to the gaseous state of a substance that is a solid or gas at room temperature. Ex. Steam is a vapor because water exists as a liquid at room temperature. Plasma the fourth state of matter and is the most abundant in the universe, but not here on earth. Stars are made up of plasma. Changes in Matter I. Physical Changes A. Definition: a change in which the same substance is present before and after the change.(its composition does not change) B. Examples: Cutting a sheet of paper, sawing a piece of wood in half, tearing lettuce, crushing ice, dissolving, all phase changes II. Chemical Changes A. Definition: a change of conditions that creates new substances with new properties B. Examples: Iron + Oxygen → rust (Iron(III) oxide) Sodium + Chlorine → table salt (NaCl) Burning a match, digesting food, silver tarnishing, iron rusting, corrosion, combustion, oxidation 2 C. Recognizing a chemical change (rules of thumb): 1. formation of a solid which is called a _precipitate_______________. 2. change in color 3. effervescence (production of a gas) 4. change in temperature 5. change in odor 6. production of light/heat III. Conservation of Mass A. Law of Conservation of Mass - mass is neither created nor destroyed during a chemical reaction. In equation form: Mass reactants = Mass products Practice Problem In an experiment, 10.00 g of red mercury (II) oxide powder is placed in an open flask and heated until it is converted to liquid mercury and oxygen gas. The liquid mercury has a mass of 9.26g. What is the mass of oxygen formed in the reaction? 2HgO → Reactant 10.00 g = 2Hg + O2 Products 9.26 g + X X = 10.00 g - 9.26 g = .74 g Mixtures of Matter I. Matter A. Definition – anything thing that takes up space and has mass. B. Matter can be broken down into two categories: mixtures and pure substances. 3 II. Mixture A. Definition: combination of two or more pure substances in which each pure substance retains its individual chemical properties. B. Examples: Sand and water; salt water C. Types of Mixtures: 1. Heterogeneous Mixture a. Definition: one that does not blend smoothly throughout and in which the individual substances remain distinct; it has two or more distinct areas. b. Examples: Sand and water, granite, raising bread, salad dressing, Orange juice (with pulp) 2. Homogeneous Mixture a. Definition: has constant (uniform) composition throughout; it always has a single phase. b. Also called a solution c. Examples: Pure air, soda(Coca Cola), vinegar, salt water, alloy III. Separating Mixtures Mixtures can be separated based on their physical properties and the techniques used can be easy or difficult. For instance: How would you separate pennies and nickels? Sorting How would you separate sand and iron filings? Magnet How would you separate salt and water? Evaporation, boiling Numerous techniques have been developed to separate mixtures besides the ones named above. Techniques 1. Sorting - by color, shape, texture, etc. 2. Magnet – separates magnetic from nonmagnetic material. 3. Density - “sink vs. float” ( Water has a density of 1 g/mL. Oil floats on water because oil's density is less than 1 g/mL) 4. Filtration - use of a porous barrier to separate solid from liquid 4 (ex. sand from salt water) 5. Distillation - Based on differences in boiling points. (2 liquids can be separated by evaporation/condensation) 6. Crystallization - Results in the formation of solid crystals from solution. 7. Chromatography - separates components of a mixtures based on the tendency of each to travel or be drawn across the surface of another material. 8. Vaporization – separates a solution. (ex. Salt from water) IV. Pure Substances Pure Substances A. Definition: Matter that has uniform and unchanging composition. B. Types of Pure Substances: 1. Element a. Definition: a pure substance that cannot be separated into simpler substances by physical or chemical means. 1) monatomic – unbonded atoms ex/ Fe, Al, Cu, He 2) diatomic – atoms not found free in nature unless bonded to an identical atom. Ex/ Br2, I2, N2, Cl2, H2, O2, F2 b. Displayed on the Periodic table. Has 18 groups – vertical columns Has 7 periods - horizontal rows 2. Compound a. Definition: combination of two or more different elements that are combined chemically. b. Examples: NaCl, SrBr2 Intro to Periodic Table I. Groups Each vertical column is called a group or family 5 II. 18 groups, numbered 1 to 18 Groups 1,2 and 13-18 are called main-group, or representative elements. They are also labeled IA, IIA and IIIA-VIIIA respectively. Groups 3-12 are called transition metals Metals, Nonmetals and Metalloids A stairstep line on the periodic table separates metals from nonmetals Metals are to the left of the stairstep line Nonmetals are to the right of the stairstep line Properties of Metals 1. good conductors of heat and electricity 2. shiny 3. have high melting points 4. ductile 5. malleable 6. tend to lose electrons Properties of Nonmetals 1. poor conductors of heat and electricity 2. dull 3. have low melting point 4. solids are brittle 5. tend to gain electrons Properties of Metalloids All elements on either side of the stairstep line EXCEPT Aluminum They have properties of both metals and nonmetals 1. white or gray in color 2. conduct heat and electricity but not as well as metals 3. ductile 4. malleable 5. not as shiny as metals 6. solids 6