RS 6
advertisement
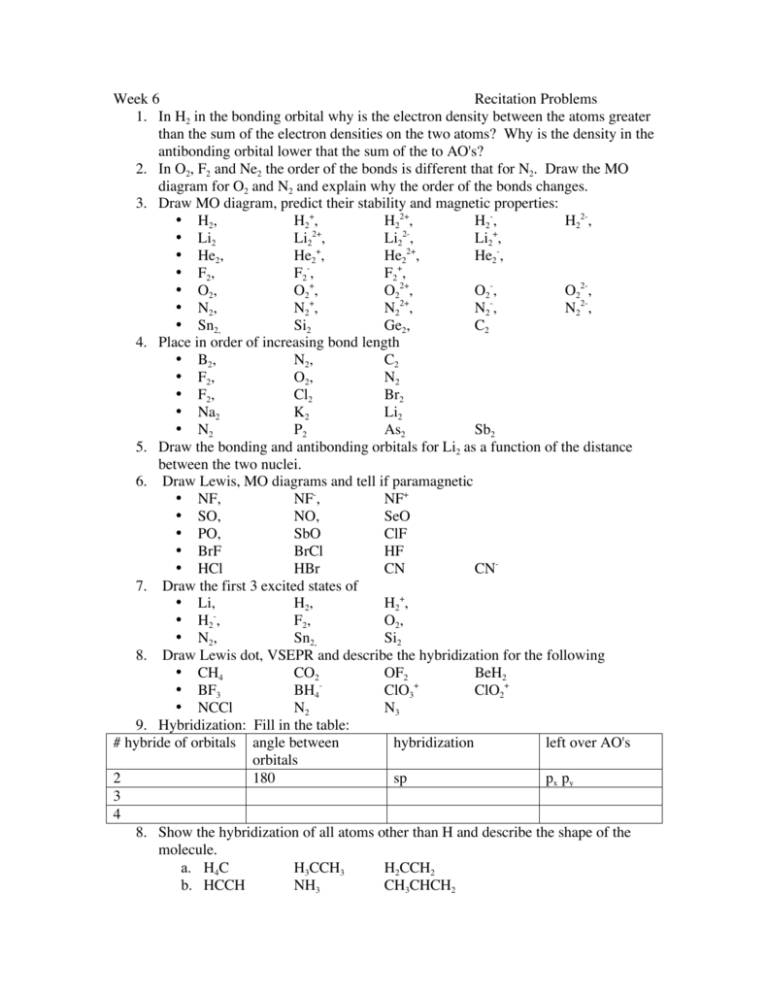
Week 6 Recitation Problems 1. In H2 in the bonding orbital why is the electron density between the atoms greater than the sum of the electron densities on the two atoms? Why is the density in the antibonding orbital lower that the sum of the to AO's? 2. In O2, F2 and Ne2 the order of the bonds is different that for N2. Draw the MO diagram for O2 and N2 and explain why the order of the bonds changes. 3. Draw MO diagram, predict their stability and magnetic properties: • H2, H2+, H22+, H2-, H22-, • Li2 Li22+, Li22-, Li2+, + 2+ • He2, He2 , He2 , He2-, • F2 , F2 - , F2+, + • O2, O2 , O22+, O2-, O22-, • N2, N2+, N22+, N2-, N22-, • Sn2, Si2 Ge2, C2 4. Place in order of increasing bond length • B2, N2, C2 • F2 , O2, N2 • F2 , Cl2 Br2 • Na2 K2 Li2 • N2 P2 As2 Sb2 5. Draw the bonding and antibonding orbitals for Li2 as a function of the distance between the two nuclei. 6. Draw Lewis, MO diagrams and tell if paramagnetic • NF, NF-, NF+ • SO, NO, SeO • PO, SbO ClF • BrF BrCl HF • HCl HBr CN CN7. Draw the first 3 excited states of • Li, H2, H2+, • H2-, F2 , O2, • N2, Sn2, Si2 8. Draw Lewis dot, VSEPR and describe the hybridization for the following • CH4 CO2 OF2 BeH2 • BF3 BH4ClO3+ ClO2+ • NCCl N2 N3 9. Hybridization: Fill in the table: # hybride of orbitals angle between hybridization left over AO's orbitals 2 180 sp px py 3 4 8. Show the hybridization of all atoms other than H and describe the shape of the molecule. a. H4C H3CCH3 H2CCH2 b. HCCH NH3 CH3CHCH2 c. CH3CHO H3CCOOH H3CCOCH3 d. H2CCHCHCH2 CS2 PF3 9. π Bonds: Straight chain hydrocarbons: Draw MO diagrams and show the AO orbital contribution, predict the relative bond lengths and shapes for: a. C6H8 H12C10 H7C5 10. π Bonds: Cyclic Hydrocarbons: Draw MO diagram, show AO contributions to MOs and predict if they are aromatic: a. C4H4 C4H42+ C4H42• + b. C5H5 C5H5 C5H5c. C8H8 C8H82+ C8H82d. C10H10 C10H102+ C10H102- Problems RS 7 1. In H2 in the bonding orbital why is the electron density between the atoms greater than the sum of the electron densities of the two atoms? When is it the same? Why is the density in the antibonding orbital lower that the sum of the to AO's? Probablity of finding an electron at a particular position is given by Ψ2 since for a bonding and antibonding orbital on H2 if we label the nuclei as Ha and Hb and the wavefunction for the H atoms as ψa and ψb we have the bonding and antibonding orbrtials as " = # a + # b ; and " * = # a $ # b . The the electron probability is given by 2 ! ! 2 " 2 = (# a + # b ) = # a 2 + # b 2 + 2# a# b ; and " *2 = (# a $ # b ) = # a 2 + # b 2 $ 2# a# b the cross terms ψaψb are due to the interfearance between the wavefunctions and are the reason that the density can be more that just the sum of the densities of the two separate orbitals. At the center point between the two nuclei the value of are due to the interfearance between the wavefunctions and are the reason that the density can be more that just the sum of the densities of the two separate orbitals. At the center point between the two nuclei the value of ψa=ψb so " 2 = 4# a = 4# b ; and " *2 = 0 2. In O2, F2 and Ne2 the order of the bonds is different that for N2. Draw the MO diagram for O2 and N2 and explain why the order of the bonds changes. ! for Homonuclear molecules MO diagram N O N O !" !" #" #" 2p 2p ! 2p 2p # # ! !" 2s 2s !" ! 2s 2s a. bond order for N (see left side picture b. Bond order for O see right side picture c. Reason is energy distance between 2s and 2p orbitals and the interaction between them d. Predict bond order, number of unpaired electrons, ! 3. Draw MO diagram, predict their stability and magnetic properties: Molecule MO diagram Bond Order # of unpaired electrons H2 above left 1 0 + H2 above left ½ 1 H22+ above left 0 0 H2 above left ½ 1 2H2 above left 0 0 Li2 above left 1 0 2+ Li2 above left 0 0 Li22above left 0 0 Li2+ He2 He2+ He22+ He2F2 F2 F2 + O2 O2+ O22+ O2O22N2 N2+ N22+ N2N22Sn2 Si2 Ge2 C2 above left above left above left above left above left Above right Above right Above right Above right Above right Above right Above right Above right Above left Above left Above left Above left Above left Above left ½ 0 ½ 1 ? 1 ½ 1½ 2 2½ 3 1½ 1 3 2½ 2 2½ 2 2 2 2 2 1 0 1 0 1 0 1 1 Para 2 Para 1 0 1 0 0 Par 1 0 Para 1 0 Para 2 ePara 2 ePara 2 ePara 2 e- 4. Place in order of increasing bond length • N2<C2<<B2 • N<O2<F2, • F2<Cl2<Br2 • Li2< Na2<K2 • N2<P2<As2<Sb2 5. Draw the bonding and antibonding orbitals for Li2 as a function of the distance between the two nuclei. !"2s !2s !"1s !1s equilibrium distance in molecule distance between nuclei 6. Draw Lewis, MO diagrams and tell if paramagnetic First need Iz of the various atoms: Atom 1s 2s 2p 3s 3p H 110 Li 44 C 157 86 4s 4p N O F P S Cl 206 261 374 106 128 151 151 167 204 NF-, NF, • (5 e- ) N F(7 82 94 111 NF+ For NF need Iz of N 206, and 106 for 2s and 2p and F is 374 and 151 for 2s and 2p. So the F2s is too low energy to bond with anything. Thus use the R 2p to bond with the N 2s and 2p. For NF get: There are 2 unpaired electrons and the BO is 2. For NF- you add one electron and get 1 unparied electron and a BO of 1½ and for NF+ you loss an electron from NF and have one uparied electron and a BO of 2½ . e- ) 2p 2p 2s For SO the S 3s and 3p are at 167 and 94 while the O 2s and 2p ae at 261 and 128. Thus the O 2p interacts with the S3s and 2p. It is similar to the 2s diagram at left but the N orbitals would be the S 3s and 2p and the F orbitals would be for O the 2s and 2p. The O 2p would be lower than they are shown. See diagram below: NO, SeO PO, SbO ClF BrF BrCl • HCl HBr 7. Draw the first 3 excited states of • Li2, H2, 2nd Excited State Li2 First Excited state Li2 !" H2+, 3rd Excited State Li2 !" #" 2p HF CN !" #" 2p 2p ! #" 2p 2p ! 2p ! # # # !" !" !" 2s 2s ! 2s 2s ! 2s 2s ! CN- (6 e- ) S O(6 e-) 2p 2p 2s 2s 3rd Excited state O2 2nd Excited state O2 First Excited state O2 !" !" #" !" #" First Excited State N2 #" Second Excited State N2 !" 2p 2p 2p 2p 2p 2p # ! # ! # ! !" !" !" #" 2p 2p 2p ! ! • H2-, 2s 2s ! 2s 2s 2s F2 , 2p ! 2p ! # # !" 2s ! #" 2p ! !" 2s !" #" # 2s Third Excited State N2 !" 2s !" 2s ! 2s 2s ! O2, • N2, Sn2, Si2 8. Draw Lewis dot, VSEPR and describe the hybridization for the following • CH4 CO2 OF2 BeH2 • BF3 BH4ClO3+ ClO2+ • NCCl N2 N3 9. Hybridization: Fill in the table: # hybride of orbitals angle between hybridization left over AO's orbitals 2 180 sp px py 3 4 11. Show the hybridization of all atoms other than H and describe the shape of the molecule. a. H4C. sp3 H3CCH3 sp3 sp3 H2CCH2 sp2 sp2 3 3 b. HCCH, sp sp NH3 sp CH3CHCH2, sp3 sp2, sp2 c. CH3CHO sp3 sp2 H3CCOOH sp3 sp2 H3CCOCH3 sp3 sp2 sp3 d. H2CCHCHCH2 CS2 PF3 12. π Bonds: Straight chain hydrocarbons: Draw MO diagrams and show the AO orbital contribution, predict the relative bond lengths and shapes for: a. C3H3• C3H3+ C3H3- C6H8 H12C10 H7C5 13. π Bonds: Cyclic Hydrocarbons: Draw MO diagram, show AO contributions to MOs and predict if they are aromatic: a. C4H4 C4H42+ C4H42• + b. C5H5 C5H5 C5H5c. C8H8 C8H82+ C8H822+ d. C10H10 C10H10 C10H102-









