AP Chemistry Chapter 6 Outline for Concepts to Know 6.1 Wave
advertisement

AP Chemistry
Chapter 6 Outline for Concepts to Know
6.1 Wave Nature of Light
Basic anatomy and vocabulary of a wave (wavelength, frequency, amplitude)
Relationship between speed, wavelength and frequency
c= 3.00x108 m/s
order of categories of electromagnetic radiation
order and approximate range of visible radiation 4 – 8(x10-7) m
6.2 Quantized Energy and Photons
Concept of smallest unit of light energy as photon – having properties of both particles and waves
Quantum as smallest possible packets or quantities of energy
Photoelectric effect – effect of changing frequency? Changing intensity?
Calculations using Planck’s constant, such as E=hv, will NOT be tested
6.3 Line Spectra and the Bohr Model
Emission spectrum of hydrogen (see p. 225) is due to energy transitions of the single electron of hydrogen
being excited to higher energy levels and then falling back down, emitting specific wavelengths of light.
Line spectra for other elements are generally more complex, but are all due to various energy transitions of
electrons moving from excited to ground (or less excited) levels
Calculations of energy states of hydrogen and other atoms will NOT be tested
Bohr model will not be specifically tested
6.4Wave Behavior of Matter
Conceptual understanding that all matter has an energy- and a wave-equivalent, but that this only has
relevance for chemistry if considering very small particles (such as electrons)
Calculations of matter waves (equation 6.8) will not be tested
Uncertainty principle as being a limit to which the momentum and position of an object can be determined –
relevance for chemistry? Electrons can be described as existing within a probability cloud around a nucleus (or
between nuclei), but can never be pinpointed.
6.5 Quantum Mechanics and Atomic Orbitals
Understand orbitals as areas of high probability for where an electron may be found, and that orbitals have
increasing energy in predictable ways
Know how to identify electrons around atoms with principle energy levels (n levels), sub-levels (s,p,d,f), orbitals
within sublevels (1,3,5,7), and spin (+/- ½) {discussed in 6.7}
Numeric values of l, ml, ms will not be tested. (i.e. Skip problems like sample problem 6.6)
6.6 Representations of orbitals
Know basic shapes of s,p,d, orbitals.
Identify meaning of figure 6.18 Radial Probability function for subsequent occurrences of of an orbital in higher
n levels
6.7 Many-electron atoms
Note the effect of more than one electron on energy of sublevels (Compare figure 6.17 with 6.24)
Spin and Pauli exclusion principle for placement of electrons
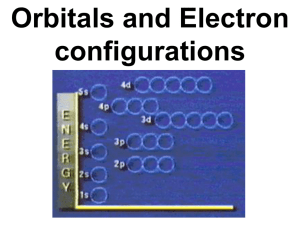
6.8 – 6.9 Electron configurations and the periodic table
Be able to complete electron configurations and orbital diagrams for ground-state multi-electron atoms or ions
Degeneracy, electron placement and Hund’s rule
Be able to write “short-cut” condensed form electron configurations; core vs. valence lectrons
Dealing with electrons in transition metals and rare earths
Anomalous electron configurations: note for chromium and copper – be able to offer explanation if oresented
with other similar