Cellular Organelles and Membrane Trafficking
advertisement

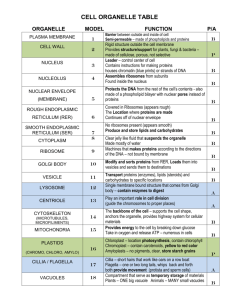
Chapter 12 Cellular Organelles and Membrane Trafficking 1 •Endomembrane System 2 •Endoplasmic Reticulum 3 •Golgi Complex 4 •Lysosomes: Endocytosis Overview 2 Includes organelles such as the endoplasmic reticulum, Golgi complex, endosomes, lysosomes, and vacuoles functioning as part of a coordinated unit. Endomembrane system 3 • Organelles of the endomembrane system are part of an integrated network in which materials are shuttled back and forth. • Materials are shuttled between organelles in membrane-bound transport vesicles. • Upon reaching their destination, the vesicles fuse with the membrane of the acceptor compartment. Overview of the Endomembrane System 4 • Biosynthetic pathway – synthesis, modification and transport of proteins. • Secretory pathway – when proteins are discharged (secreted) from the cell. • Constitutive secretion – in a continuous fashion. • Regulated secretion – in response to a stimulus. Distinct pathways through the cytoplasm 5 During regulated secretion, materials to be secreted are stored in large, membranebound secretory granules. Endomembrane System 6 Autoradiography Green Fluorescent Protein (GFP) Biochemical analysis of subcellular fractions Cell Free Systems Mutant Study Approaches to study Cytomembranes 7 • A method to visualize biochemical processes using radiolabeled materials exposed to a photographic film. • Can be used to determine where secretory proteins are synthesized by using labeled amino acids. Autoradiography 8 • A small protein isolated from jellyfish which emits green fluorescent light. • A GFP-DNA chimera allows to observe the protein synthesis in the cell. • Viral genes fused to GFP allows the study of protein traffic due to large production of proteins Use of Green Fluorescent Protein 9 • Techniques to homogenize cells and isolate some organelles, which can then be separated from one another through subcellular fractionation. • Membrane vesicles derived from the endomembrane system form a collection of vesicles called microsomes, which can be characterized further through other techniques. Biochemical Analysis of Subcellular Fractions 10 • Cell-free systems do not contain whole cells and have provided information about the roles of the proteins involved in membrane trafficking. • Other type of information identified includes: proteins that bind to the membrane to initiate vesicle formation, and proteins responsible for cargo selection. Use of Cell-Free Systems 11 • Mutants provide insights about the function of normal gene products. • Isolation of proteins from yeast has led to the identification of homologous proteins in mammals, pointing to the conserved nature of endomembrane systems. • Scientists can identify genes involved in a particular process by determining which siRNAs interfere with that process. Study of Mutants 12 ENDOPLASMIC RETICULUM 13 two components: • Rough endoplasmic reticulum (RER) • Smooth endoplasmic reticulum (SER) • Different types of cells have different ratios of the two types of ER, depending on activities of the cell. Endoplasmic reticulum (ER) 14 • Composed of a network of flattened sacs (cistenae). • Continuous with the outer membrane of the nuclear envelope and also has ribosomes on its cytosolic surface. RER 15 • Extensively developed in a number of cell types. • Functions include: • Synthesis of steroid hormones in endocrine cells. • Detoxification in the liver of various organic compounds. • Sequestration of calcium ion into cytoplasm of muscle cells. SER 16 • Polypeptides synthesized on ribosomes of RER include secreted proteins, integral membrane proteins, and soluble proteins of organelles. • Polypeptides synthesized on “free” ribosomes include one-third of those encoded by the human genome, cytosolic proteins, peripheral membrane proteins, nuclear proteins, and proteins incorporated into chloroplasts, mitochondria, and peroxisomes. Synthesis of Proteins on Membrane-Bound versus Free Ribosomes 17 • A signal sequence at N-terminus in secretory proteins. • Polypeptide moves into ER cisternal space through a protein-lined pore. • Movement through the membrane can occur as it is being synthesized (cotranslationally) or postranslationally. Site of synthesis of a protein determined by sequence of amino acids in N-terminus. 18 Synthesis of protein on membrane-bound ribosome 19 • Messenger RNA binds to free ribosomes on cytosol. • Secretory proteins synthesized on membranebound ribosomes have their signal sequence recognized by a signal recognition particle (SRP) • The SRP must interact with a SRP receptor. • Another interaction must occur between the ribosome and the translocon, which is a protein-lined channel. • Once the SRP-ribosome-nascent peptide chain complex binds to the ER, the SRP is released Synthesis of protein on membrane-bound ribosome 20 • Upon entering the RER lumen, the signal sequence is cleaved by a signal peptidase. • Carbohydrates are added by the enzyme oligosaccharltransferase. • The RER lumen is packed with chaperones to assist in folding, and also contains protein disulfide isomerase to add disulfide bonds to cysteines. Processing of Newly Synthesized Proteins in the ER 21 • Integral proteins contain hydrophobic transmembrane segments that interfere transfer into the RER lumen. • The translocon assists in the proper orientation of transmembrane sequences. • The arrangement within the membrane is determined by the orientation of the first transmembrane segment is inserted. Synthesis of Integral Membrane Proteins 22 • Addition of sugars is catalyzed by glycosyltransferases. • Core carbohydrate is modified by oligosaccharyltransferase as the polypeptide is transferred into the ER lumen. • A glycoprotein goes through a system of quality control to determine its fitness for a specific compartment. • Misfolded proteins are tagged by a terminal glucose and recognized by chaperones for refolding. Glycosylation in the RER 23 • Misfolded proteins are not destroyed in the ER; instead they are transported into the cytosol where they are destroyed in proteasomes. • This process is called ER-associated degradation (ERAD), and ensures the misfolded proteins do not reach the cell surface. • Accumulation of misfolded proteins triggers the unfolded protein response (UPR). Destruction of misfolded proteins 24