Diameter of a Molecule
advertisement

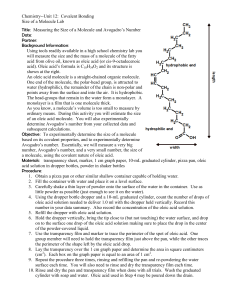
Title: The Diameter of a Molecule Purpose: To estimate the diameter of a single molecule of oleic acid. So that you can determine for yourself the extreme smallness of a molecule. How it works. Oleic acid is an organic acid soluble in alcohol but insoluble in water. When the acid is placed in water it usually spreads out over the water surface creating a mono-layer (a layer one molecule thick). A single drop of this acid could easily cover the surface of a large swimming pool. This is the reason for using a very weak solution of the acid in this laboratory. Lab Equipment: 1. Large tray 3. One drop of oleic acid in alcohol (concentration 5 ml of acid per 1000 ml of solution) 2. Ruler or meter stick 4. Lycopodium Powder 5. 10 ml graduated cylinder A=πr2 V = Ah Procedure: 1. Pour water into a large tray so that the bottom of the tray is completely covered with water. Lycopodium powder is in the salt shakers. Shake lightly to spread the powder over the surface of the water so to form a very, very thin layer. 2. Using a pipet gently add a single drop of the oleic acid solution to the surface of the water. When the drop touches the water, the alcohol will dissolve in the water but the acid in the drop will not. Instead, it will spread out into a roughly circular pattern. Carefully measure the diameter of the acid area in cm. Since the expanse is not a true circle, measure its diameter in several places and calculate the average diameter of the expanse. 3. The concentration of oleic acid in the solution is 5 ml per 1000 ml of solution. This means that every ml of the solution contains 5/1000 ml or 5.0 X 10-3 ml of acid. If you could determine the volume of the single drop you could determine the volume of oleic acid in the drop. This, in turn, would be the volume of the expanse of acid in the tray. (back) 4. To determine the volume of a single drop of solution use the 10 ml graduated cylinder and the pipet. Carefully count the number of drops of water needed to occupy 2 ml in the cylinder. Do this 3 times to be sure of the number of drops and take the average. Remember to read the bottom of the meniscus. 5. Divide 2 ml by the number of drops in 2 ml to determine the volume of a single drop. Multiply this volume by 5 X 10-3 to find the volume of oleic acid in the drop. This is the volume of the layer of acid in the tray. Conclusion Questions: 1. Calculate the area of the layer. 2. The layer constitutes a cylinder one molecule in height. Calculate the height of the cylinder. This is the diameter of the oleic acid molecule. 3. Assume that an oleic acid molecule is a cube. Determine the volume of an oleic acid molecule. 4. Using your estimate of the volume of an oleic acid molecule, determine the number of oleic acid molecules in 1 ml (cm3) of the acid. 5. The density of oleic acid is 0.9 g/ml. Using your value for the volume of a single oleic acid molecule determine the mass of one molecule of the acid. 6. Now calculate the molecular weight of a mole of oleic acid molecules. 7. One mole (the molecular weight in grams) of oleic acid has a mass of 282 g. Calculate how many molecules of oleic acid would be contained in a mole of the acid? This should give Avogadro’s number but probably does not. However, is the result within the same order of magnitude as Avogadro’s number? Title: Name: _________________________ Purpose: Data: (don’t forget units) 1. Average diameter of circle in cm. _________ 2. Number of drops to fill 2 ml. _____ _____ _____ average ______ 3. Volume of the layer of acid in the tray. __________ Conclusion Questions: 1. Area of the layer _________ 2. Height of the cylinder and diameter of one molecule. _________ 3. Volume of oleic acid molecule. ___________ 4. Number of oleic acid molecules in 1 ml of the acid. __________ 5. Mass of one molecule. __________ 6. Molecular weight of a mole of oleic acid molecules. ____________ 7. What was your calculated molecules of 1 mol. ___________