Unit 12 Size of a Molecule Lab
Chemistry--Unit 12: Covalent Bonding
Size of a Molecule Lab
Title
: Measuring the Size of a Molecule and Avogadro’s Number
Date
:
Partner :
Background Information
:
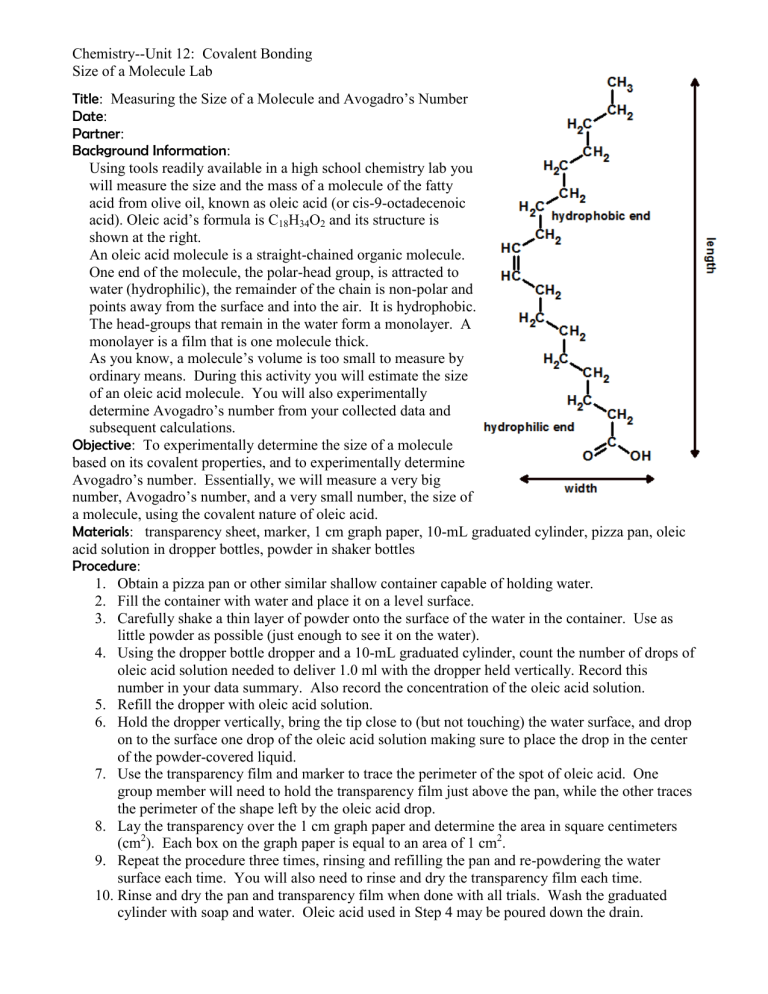
Using tools readily available in a high school chemistry lab you will measure the size and the mass of a molecule of the fatty acid from olive oil, known as oleic acid (or cis-9-octadecenoic acid). Oleic acid’s formula is C
18
H
34
O
2
and its structure is shown at the right.
An oleic acid molecule is a straight-chained organic molecule.
One end of the molecule, the polar-head group, is attracted to water (hydrophilic), the remainder of the chain is non-polar and points away from the surface and into the air. It is hydrophobic.
The head-groups that remain in the water form a monolayer. A monolayer is a film that is one molecule thick.
As you know, a molecule’s volume is too small to measure by ordinary means. During this activity you will estimate the size of an oleic acid molecule. You will also experimentally determine Avogadro’s number from your collected data and subsequent calculations.
Objective
: To experimentally determine the size of a molecule based on its covalent properties, and to experimentally determine
Avogadro’s number. Essentially, we will measure a very big number, Avogadro’s number, and a very small number, the size of a molecule, using the covalent nature of oleic acid.
Materials
: transparency sheet, marker, 1 cm graph paper, 10-mL graduated cylinder, pizza pan, oleic acid solution in dropper bottles, powder in shaker bottles
Procedure
:
1.
Obtain a pizza pan or other similar shallow container capable of holding water.
2.
Fill the container with water and place it on a level surface.
3.
Carefully shake a thin layer of powder onto the surface of the water in the container. Use as little powder as possible (just enough to see it on the water).
4.
Using the dropper bottle dropper and a 10-mL graduated cylinder, count the number of drops of oleic acid solution needed to deliver 1.0 ml with the dropper held vertically. Record this number in your data summary. Also record the concentration of the oleic acid solution.
5.
Refill the dropper with oleic acid solution.
6.
Hold the dropper vertically, bring the tip close to (but not touching) the water surface, and drop on to the surface one drop of the oleic acid solution making sure to place the drop in the center of the powder-covered liquid.
7.
Use the transparency film and marker to trace the perimeter of the spot of oleic acid. One group member will need to hold the transparency film just above the pan, while the other traces the perimeter of the shape left by the oleic acid drop.
8.
Lay the transparency over the 1 cm graph paper and determine the area in square centimeters
(cm
2
). Each box on the graph paper is equal to an area of 1 cm
2
.
9.
Repeat the procedure three times, rinsing and refilling the pan and re-powdering the water surface each time. You will also need to rinse and dry the transparency film each time.
10.
Rinse and dry the pan and transparency film when done with all trials. Wash the graduated cylinder with soap and water. Oleic acid used in Step 4 may be poured down the drain.
Chemistry--Unit 12: Covalent Bonding
Size of a Molecule Lab
Data Summary
:
Number of drops of Oleic Acid Solution to equal 1.0 ml = ______________
Concentration of Oleic Acid Solution = _____________
Results
:
Area of clear area:
Measurement 1 = ______________cm
Measurement 2 = ______________cm
2
2
Measurement 3 = ______________cm
2
Average Area = ______________cm
2
1.
Calculate the volume of pure oleic acid used using these steps: a.
Convert the 1 drop of solution used to mL. b.
Convert the mL of solution to mL of pure oleic acid. Note that a 0.20% solution means that there at 0.20 mL of pure oleic acid contained in every 100 mL of solution.
2.
Calculate the thickness (or height) of the layer of oleic acid on the water. Recall that volume can be calculated by multiplying area × height.
3.
Assuming that the oleic acid has formed a layer that is one molecule thick, and given that the width (diameter) of an oleic acid molecule is 1/10 th
its height, what is the diameter of an oleic acid molecule?
4.
With the above information, determine the number of molecules in the layer (total area = area of one molecule × number of molecules in the layer). To do this, you will need to first find the area of one molecule using the equation for the area of a circle (area circle
= π• r
2
).
5.
Given that the density of oleic acid is 0.89 g/mL, what was the mass of pure oleic acid used?
6.
From the calculations above, calculate the mass of one molecule of oleic acid.
7.
Given that the molar mass of oleic acid is 282.47 g/mol, how many moles of pure oleic acid were used?
8.
From the calculations above (#4 and #7), calculate an experimental value for Avogadro’s number.
Conclusion
: (see laboratory write-up guide sheet; be sure to include a real-world example)
Discussion of Error
: Calculate a percent error for your results for Avogadro’s number. Discuss the accuracy of these results.
Suggestions for Improvement
: (Always suggest something!)











