Topic 10: Organic Chemistry P1: …….. / 15p. P2: ……. / 29p.
IB2 HL CHEMISTRY
Topic 10: Organic Chemistry
Name: ……………………………
P1: …….. / 15p. P2: ……. / 29p.
7: 35
6: 31
5: 26
4: 22
3: 18
2: 13
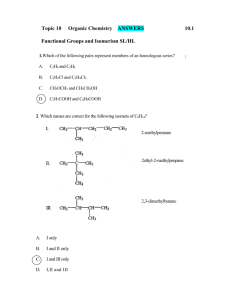
1.
Which compound can exist as optical isomers?
A. H
2
NCH
2
COOH
B. H
3
CCONH
2
C. H
3
CCHBrI
D. HCOOCH
3
2.
What is the organic product of the reaction between ethanol and ethanoic acid in the presence of sulfuric acid?
A. CH
3
CHO
B. CH
3
COOCH
3
C. CH
3
CH
2
COOCH
3
D. CH
3
COOCH
2
CH
3
3.
What is the correct IUPAC name for the following compound?
CH
3
A. 4-methylbutanenitrile
B. 4-methylpentanenitrile
C. 2-methylbutanenitrile
D. 2-methylpentanenitrile
4.
What is the product of the following reaction?
CH
3
CH
2
CH
2
CN + H
2
A. CH
3
CH
2
CH
2
NH
2
B. CH
3
CH
2
CH
2
CH
3
C. CH
3
CH
2
CH
2
CH
2
CH
3
D. CH
3
CH
2
CH
2
CH
2
NH
2
5.
Propane, C
3
H
8
, undergoes incomplete combustion in a limited amount of air. Which products are most likely to be formed during this reaction?
A. Carbon monoxide and water
B. Carbon monoxide and hydrogen
C. Carbon dioxide and hydrogen
D. Carbon dioxide and water
1
6.
Which amino acid can exist as optical isomers?
A.
B.
C.
D.
7.
How many chiral carbon atoms are present in a molecule of glucose?
A. 1
B. 2
C. 3
D. 4
2
8.
Nylon is a condensation polymer made up of hexanedioic acid and 1,6-diaminohexane.
Which type of linkage is present in nylon?
A. Amide
B. Ester
C. Amine
D. Carboxyl
9.
Which species reacts most readily with propane?
A. Br
2
B. Br•
C. Br
–
D. Br
+
10.
Which pair of compounds can be used to prepare CH
3
COOCH
3
?
A. Ethanol and methanoic acid
B. Methanol and ethanoic acid
C. Ethanol and ethanoic acid
D. Methanol and methanoic acid
11.
Which compound forms when hydrogen bromide is added to but-2-ene?
A. 2-bromobutane
B. 2,3-dibromobutane
C. 1-bromobutane
D. 1,2-dibromobutane
12.
The following is a three-dimensional representation of an organic molecule.
Which statement is correct?
A. The correct IUPAC name of the molecule is 2-methylpentane.
B. All the bond angles will be approximately 90°.
C. One isomer of this molecule is pentane.
D. The boiling point of this compound would be higher than that of pentane.
3
13.
Which statement about successive members of all homologous series is correct?
A. They have the same empirical formula.
B. They differ by a CH
2 group.
C. They have the same physical properties.
D. They differ in their degree of unsaturation.
14.
An organic compound X reacts with excess acidified potassium dichromate(VI) to form compound Y , which reacts with sodium carbonate to produce CO
2
(g).
What is a possible formula for compound X ?
A. CH
3
CH
2
COOH
B. CH
3
CH
2
CH
2
OH
C. CH
3
CH(OH)CH
3
D. (CH
3
)
3
COH
15.
Which compound is a tertiary halogenoalkane?
A. (CH
3
CH
2
)
2
CHBr
B. CH
3
(CH
2
)
3
CH
2
Br
C. (CH
3
)
2
CHCH
2
CH
2
Br
D. CH
3
CH
2
C(CH
3
)
2
Br
16.
The compound, 2-bromobutane, CH
3
CHBrCH
2
CH
3
, can react with sodium hydroxide to form compounds F , G and H .
Compound F , C
4
H
10
O, exists as a pair of optical isomers. Compounds G and H , C
4
H
8
, are structural isomers, and compound H exists as a pair of geometrical isomers.
(i) Draw the structures of the two optical isomers of F .
(2)
4
(ii) Outline the use of a polarimeter in distinguishing between the optical isomers.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
(2)
(iii) Draw diagrams to show the shapes of the two geometrical isomers of H .
(iv) Draw the mechanism, using curly arrows to represent the movement of electron pairs, to show the formation of G .
(3)
(Total 9 marks)
(2)
5
17.
The molecular formula, C
3
H
4
Cl
2
represents several isomeric compounds. Some isomers are cyclic and some are unsaturated.
(a) Draw the structures of two cyclic compounds that are structural isomers and state the names of both isomers.
(2)
(b) Two of the non-cyclic compounds have geometrical isomers. Draw the structures of these compounds and their geometrical isomers.
18.
(i) Identify the formulas of the organic products, A–E, formed in the reactions, I – IV :
(2)
(Total 4 marks)
I.
CH
3
(CH
2
)
8
OH + K
2
Cr
2
O
7
A
B
II.
(CH
3
)
3
CBr + NaOH C
III.
(CH
3
)
2
CHOH + K
2
Cr
2
O
7
D
IV.
H
2
C=CH
2
+ Br
2
E
(5)
6
(ii) H
2
C=CH
2
can react to form a polymer. Name this type of polymer and draw the structural formula of a section of this polymer consisting of three repeating units.
(2)
(Total 7 marks)
19.
The following is a computer-generated representation of the molecule, methyl
2-hydroxy benzoate, better known as oil of wintergreen.
(i) Deduce the empirical formula of methyl 2-hydroxy benzoate and draw the full structural formula, including any multiple bonds that may be present.
The computer-generated representation shown does not distinguish between single and multiple bonds.
(2)
7
(ii) In this representation, two of the carbon-oxygen bond lengths shown are 0.1424 nm and
0.1373 nm. Explain why these are different and predict the carbon-oxygen bond length in carbon dioxide.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
(2)
(iii) Name all the functional groups present in the molecule.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
……………………………………………………………………………………………………
(2)
(Total 6 marks)
20.
Polyesters are formed in a condensation reaction. The structure of the repeat unit of a polyester is
C C H C O CH CH O
O O
(i) Draw the structures of the two monomers that react to form this polyester.
(2)
(ii) Identify the essential feature of the monomers in (i) that enable them to form a condensation polymer.
……………………………………………………………………………………………………
……………………………………………………………………………………………………
(1)
(Total 3 marks)
8
10.
8.
9.
4.
5.
6.
7.
1.
2.
3.
C
D
B
D
A
B
D
A
B
B
[1]
[1]
[1]
[1]
[1]
[1]
[1]
[1]
[1]
[1]
9
14.
15.
11.
12.
13.
16.
A
C
B
B
D
(i)
OH OH
(ii)
(iii)
CH CH
2
C
H H
C
CH
3
CH
3
3
Award [2] for both tetrahedral structures, or [1] if tetrahedral structure is not clear. plane polarized light; rotation in opposite/different directions;
CH
3
CH
3
C C ;
H H
(iv)
CH
3
H
C C ;
H CH
3 curly arrow showing attack by
–
OH on end H; curly arrow showing C–Br bond fission; curly arrow showing formation of double bond;
H2O and Br
–
shown as products;
Award [1] each for any three.
If but-2-ene formed, award [2 max] .
17.
(a)
Cl Cl and 1,1 dichlorocyclopropane;
Cl Cl Cl Cl
Cl Cl Cl
( cis - or trans -) 1,2 dichlorocyclopropane;
Award point for the correct name corresponding to the related isomer.
Accept diagrams that do not display 3 dimensional structure.
Award [1 max] for correct structures only, without the corresponding names.
Cl
2
2
2
2
3 max
[9]
10
[1]
[1]
[1]
[1]
[1]
(b)
Cl CH
3
Cl and
H Cl H
Cl Cl and
H H H
18.
(i)
(ii)
A. = CH3(CH2)7CHO;
B. = CH3(CH2)7COOH/CH3(CH2)7CO2H;
C. = (CH3)3COH;
D. = (CH3)2CO;
E. = BrCH2CH2Br;
Allow correct structural formulas. addition;
Cl
CH
3
H
/-(CH2-CH2)3-/-(CH2)6-;
2
5
2
19.
20.
(i)
(ii)
(i) (Empirical formula =) C8H8O3;
H
H O
O
H C C H
H
O
;
H H
H
Allow double bonds on arene in alternate positions, or allow delocalized representation
(of pi electrons).
(ii) the bond at 0.1373 nm is a double bond and the bond at 0.1424 nm is a single bond; in CO2(g) both bonds are double bonds and would have a value around 0.137 nm;
(iii) Ester;
Arene/benzene ring;
Alcohol;
Award 2 for any three correct, award [1] for any two correct.
Do not accept alkane as a type of functional group in this molecule.
HOCH2CH2OH;
HOOCC6H4COOH; reactants have two functional groups/ OWTTE ;
2
2
2
2
1
[4]
[7]
[6]
[3]
11