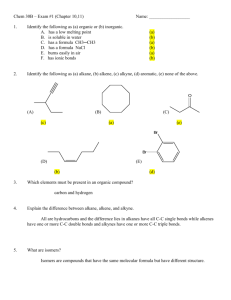
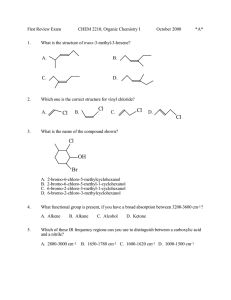
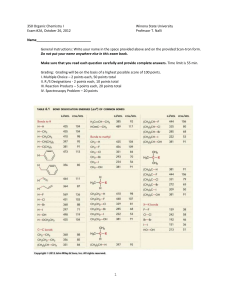
Experiment 1: Melting Point
advertisement
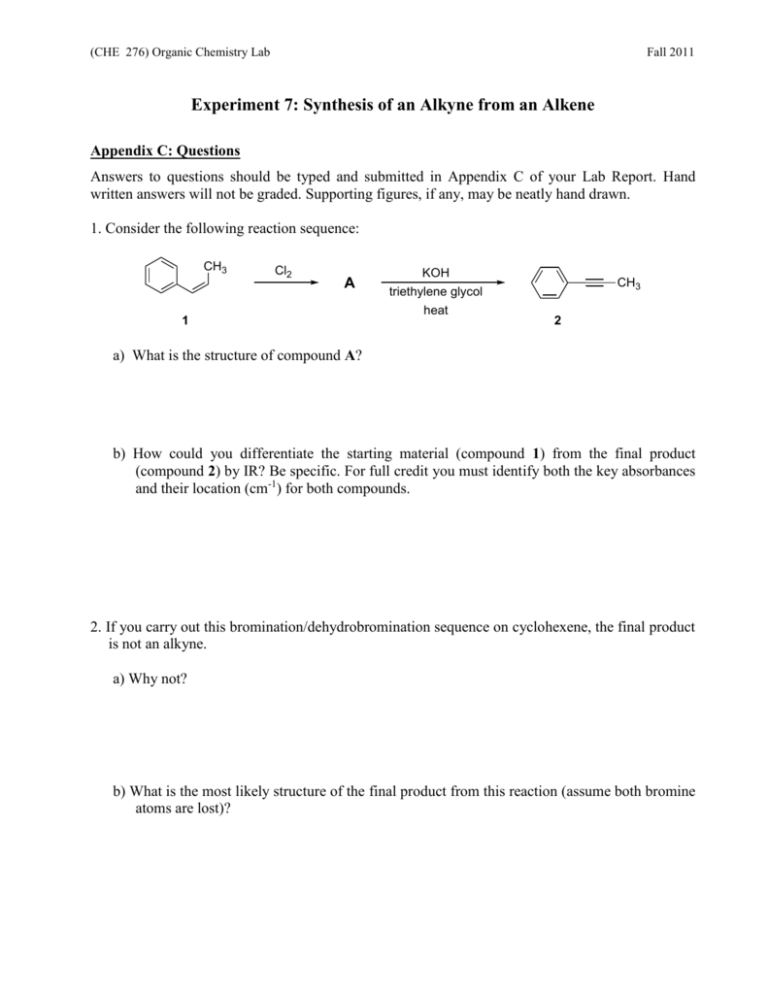
(CHE 276) Organic Chemistry Lab Fall 2011 Experiment 7: Synthesis of an Alkyne from an Alkene Appendix C: Questions Answers to questions should be typed and submitted in Appendix C of your Lab Report. Hand written answers will not be graded. Supporting figures, if any, may be neatly hand drawn. 1. Consider the following reaction sequence: CH3 Cl2 A 1 KOH CH3 triethylene glycol heat 2 a) What is the structure of compound A? b) How could you differentiate the starting material (compound 1) from the final product (compound 2) by IR? Be specific. For full credit you must identify both the key absorbances and their location (cm-1) for both compounds. 2. If you carry out this bromination/dehydrobromination sequence on cyclohexene, the final product is not an alkyne. a) Why not? b) What is the most likely structure of the final product from this reaction (assume both bromine atoms are lost)?