The 3-D Structure of Molecules
advertisement

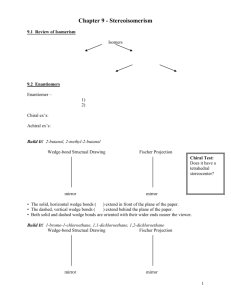
The 3-D Structure of Molecules - Stereochemistry For additional help, check out the Organic Chemistry section of the following website: http://www.khanacademy.org/#browse Scroll down to the items on “Chirality”, etc. Isomerism Consitutional isomers - Atoms are bonded to different atoms - different IUPAC names & structural formulas Structural Positional Functional group Stereoisomers Atoms are oriented differently Cis-Trans - orientation around a double bond Tetrahedral C atom mirror images Chiral vs. Achiral Chirality Chirality is based on whether a molecule has a “chiral” center. Carbon atom with FOUR DIFFERENT attached (tetrahedrally) groups Chiral molecules = mirror images that are NOT superimposable vs. •(left right handedness) Achiral molecules = mirror images that are superimposable vs. •(no left right handedness) Left or right handedness of monosaccharides is determined by the position of -OH on the chiral center. Naturally occurring monosaccharides are almost always right-handed. Plants produce only right-handed monosaccharides Stereoisomerism - molecules that have the same molecular AND structural formulas but different orientation of atoms In order for molecules to exhibit stereoisomerism they must have: A chiral center Structural rigidity This is the basis for cis-trans isomerism Two types of Stereoisomer Enantiomers - molecules that are nonsuperimposable mirror images Ex.: Left & right handed with single chiral center Diastereomers - molecules that are not mirror images Ex.: Cis-trans (possible in some rings and around double bonds Thalidomide - an example of a chiral center in a cyclic compound Fischer Projection - 2-dimensional structural notation showing the spatial arrangement of groups around chiral centers (to show handedness) Tetrahedral geometry: Vertical lines = bonds directed into the page Horizontal lines = bonds directed out of the page Carbon chain is positioned vertically, with the carbonyl group at or near the top. Ex.: glyceraldehyde (2,3-dihydroxypropanal) Latin: –Dextro = Right –Levo= Left Determine “D” vs “L” by examining the position of the functional group on the chiral center Compounds with multiple chiral centers Naming is complex Use the highest # chiral C atom in the chain to determine “D” or “L”. If there are 2 or more “D”s and 2 or more “L”s, use different common names for each pair. Ex. 2,3,4-trihydroxybutanal Number of Stereoisomers possible for a particular molecule: General rule* # of isomers = 2n (n = # of chiral centers) *Sometimes symmetry considerations make some mirror images superimposable. Properties of Isomers Constitutional - differ in most physical and chemical properties Diastereomers - differ in most physical and chemical properties Enantiomers - differ in only two properties: Interactions with plane-polarized light (ppl) Interactions with other chiral substances Dextrorotatory & Levorotatory Compounds An enantiomer (chiral cpd) that rotates “ppl” in a clockwise direction is dextrorotatory. (+) An enantiomer (chiral cpd) that rotates “ppl” in a counterclockwise direction is levorotatory. (-) The handedness of enantiomers and the direction of rotation are, unfortunately, not related. Interactions Between Chiral Compounds Enantiomers have the same FP, BP, density, etc. Properties depend on IMF IMF does not depend on Chirality IMF depends on functional groups Enantiomers have the same solubility in achiral solvents (ethanol), but different solubility in chiral solvent (D-2-butanol). Rate & Extent of Reaction of Enantiomer is the same with an achiral reactant but different with another chiral reactant. Receptor sites for molecules in the body have chirality, so enantiomers generate different responses. Enantiomers react differently to taste buds: spearmint vs.carroway D-Epinephrine binds to the receptor at three points. The human body exhibits a response to the D form that is 20 times greater than the repsonse to the L form.