2 - FM's Chem Depot
advertisement

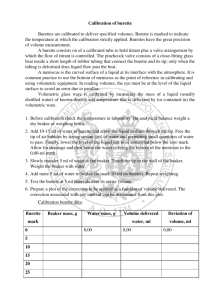
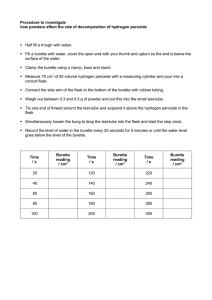
UNIT 2 MODULE 2 CALIBRATION OF A BURETTE Skills tested: None INTRODUCTION: In this experiment, you will accurately determine the actual volume delivered by a burette with readings of 5 ml, 15 ml, 25 ml and 35 ml. Without calibration, a systematic error of unknown magnitude may exist. PROCEDURE 1. Collect distilled water in a beaker and let it stand for about 15 minutes before measuring and recording the temperature of the water. 2. Weigh the dry empty beaker on the balance. 3. Using a small filter funnel, fill the burette to the zero mark. Allow 20 seconds to pass and re-read the burette to ensure the bottom of the meniscus has not moved. 4. Dispense exactly 5 ml of water in the beaker and measure and record the mass of the beaker or container. ENSURE THE BOTTOM OF THE BEAKER HAS BEEN DRIED THOROUGHLY BEFORE WEIGHING IT. Repeat a second time for the second trial at 5 ml. 5. Use the data in table 1 below to convert the mass of water collected to volume for both trials at 5 ml and find the difference between the apparent volume (volume dispensed) and the true volume (volume calculated from mass) which is called the correction factor and then find the average correction factor. (Use MS Excel or plot the graph by hand in order to determine the density of the water you have used, include this graph in your report) 6. Collect a fresh clean dry beaker and repeat steps 1 - 5 for 15 ml, 25 ml and 35 ml. 7. Determine the overall average correction factor for the burette you have used. 8. Do not write a full lab report, complete the results table below and answer the questions which follow. TABLE 1 Density of water at various temperature X T/oC 26 28 30 Y Density /g cm-3 0.99678 0.99623 0.99565 32 0.99503 34 0.99438 36 0.99369 Results & Calculations Temperature of water Apparent volume Mass of water /g Density of water Calculated volume Correction factor (- or +) Average correction factor Overall average correction factor ........................ Trial 1 at 5 ml 5 ml Trial 2 at 5 ml 5 ml Trial 1 at 15 ml 15 ml Trial 2 at 15 ml 15 ml Trial 1 at 25 ml 25 ml Trial 2 at 25 ml 25 ml Trial 1 at 35 ml 35 ml Trial 2 at 35 ml 35 ml Treatment of results 1. What type of burette did you use? Class A or Class B? Based on the type of burette used, state the tolerance or accuracy of the burette. 2. The existence of a correction factor implies that error was being incurred each time a burette was used for a titration, what type of error would this signify? Discuss sources of this type of error you have just named.