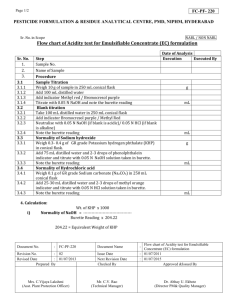
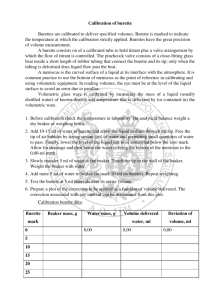
Review of Chemistry 11 Practice Test 2
advertisement
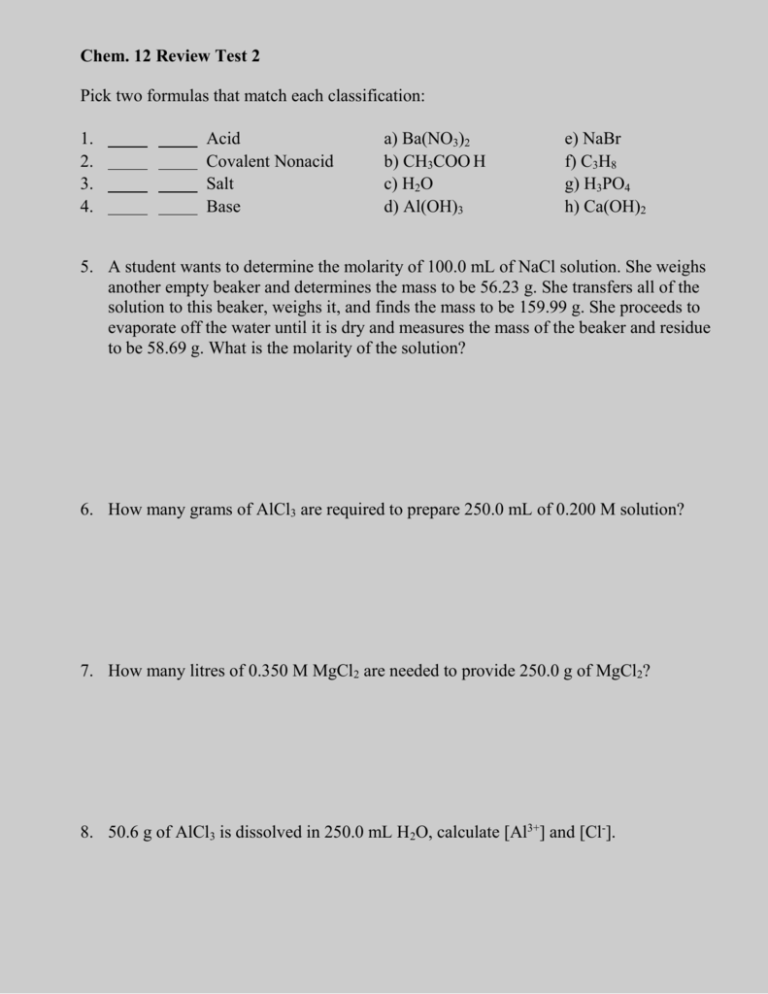
Chem. 12 Review Test 2 Pick two formulas that match each classification: 1. 2. 3. 4. Acid Covalent Nonacid Salt Base a) Ba(NO3)2 b) CH3COO H c) H2O d) Al(OH)3 e) NaBr f) C3H8 g) H3PO4 h) Ca(OH)2 5. A student wants to determine the molarity of 100.0 mL of NaCl solution. She weighs another empty beaker and determines the mass to be 56.23 g. She transfers all of the solution to this beaker, weighs it, and finds the mass to be 159.99 g. She proceeds to evaporate off the water until it is dry and measures the mass of the beaker and residue to be 58.69 g. What is the molarity of the solution? 6. How many grams of AlCl3 are required to prepare 250.0 mL of 0.200 M solution? 7. How many litres of 0.350 M MgCl2 are needed to provide 250.0 g of MgCl2? 8. 50.6 g of AlCl3 is dissolved in 250.0 mL H2O, calculate [Al3+] and [Cl-]. 9. 600.0 mL of 0.200 M H2SO4 reacts with 600.0 mL of 0.200 M NaOH. Calculate concentration of the excess acid in the new solution. 10. In three runs of a titration 0.200 M NaOH was used to neutralize a 25.0 mL sample of H2CO3. Calculate the molarity of the acid. 0.200 M NaOH in the burette Initial burette reading (mL) Final burette reading (mL) 2.05 10.56 10.56 19.09 19.09 27.80 11. How many grams of .0200 M H2C2O4 are required to neutralize 250.0 mL of .0250 M KOH? 12. Complete the reaction equations. i) Formula Equation/Chemical Equation Sr(OH)2 (aq) + ZnSO4 (aq) ii) Total Ionic Equation iii) Net Ionic Equation 13. Write the complete ionic equation for the reaction of Mg (s) and HCl (aq). 14. What volume of 0.100 M H2SO4 is needed to neutralize 25.0 mL 0.250 M NaOH and 30.0 mL of 0.200 M KOH solution? 15. Calculate all ion concentrations after 200.0 mL of 0.200 M CaCl2 is mixed with 200.0 mL of 0.300 M AlCl3. 16. What concentration of acid or base remains when 200.0 mL of 0.200 M H2SO4 is mixed with 200.0 mL 0.100 M KOH and 400 mL 0.100 M NaOH.