CHEMISTRY 1A
advertisement

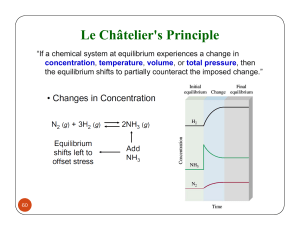
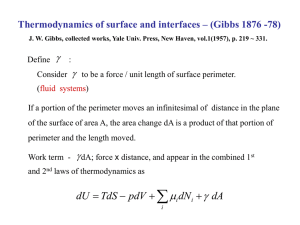
100 Points CHEMISTRY 1A Exam 5 Name___________________________ SHOW ALL OF YOUR WORK! 1. Draw an appropriate Lewis structure for CO22- and for CO32-. a. Give the bond order for the C-O bond in each ion. b. Which has the weakest bonds? c. Which has the shortest bonds? (6) (2) (2) (2) 2. Draw the structure with no double bonds and calculate the formal charge and oxidation number for each atom. (8) IO2Cl2 (I is the central atom) 3. Draw a 3-D diagram for each molecule and indicate if the molecule is polar or non-polar. a. SiF4 b. OF2 (6) 4. Determine the molecular shape for each. Give the VSEPR class, draw the 3-D structure, and provide an appropriate VSEPR name for the shape. (20) a. IF4b. SeO2 c. XeF3+ d. SCl4 5. Calculate the enthalpy of reaction using bond energies. Use the table of average bond energies provided. (8) H H H C C H H O Single Bond Energies (kJ/mole) 1A 4A 5A 6A H C N O H 436 413 391 436 C 346 305 358 N 163 201 O 146 H + OF 2 H H O C C O H + 2HF H 7A F 565 485 283 190 Multiple Bond Energies (kJ/mole) C=C 602 CC 835 C=N 615 CN 887 C=O 732 CO 1072 6. At 432C, KP = 6.7103 for the reaction: 2 NH3(g) NH3(g) Calculate KP for the reaction: 3 H2(g) + N2(g) (6) 3/2 H2(g) + 1/2 N2(g) 7. At 1050 K, KC = 1.25. The system is analyzed and these concentrations are found: [O2] = 2.45 M, [NO] = 3.15 M, [NO2] = 1.95 M. 2 NO(g) + O2(g) 2 NO2(g) a. Is the system in equilibrium? SHOW YOUR WORK! (5) b. If "No" above, which way does the system shift to reach equilibrium, and why? (5) 8. At 312C, KP = 12.2 for the reaction: PCl5(g) PCl3(g) + Cl2(g) If 0.075 mole of reactant is placed in a 5.0 L flask and heated to 312C, what is the equilibrium pressure for each gas? (10) 9. An experiment shows that at 15C and a total pressure of 16.0 atm., the equilibrium ratio of Z to T, in moles, is exactly 5 to 3 respectively. Calculate KP and KC for the reaction. (10) 9 Z(g) 4 T(g) 10. At a certain temperature, 7.68 atm. of O2(g) is placed in a 20.0 liter container with 15.24 atm. of NH3(g) and allowed to come to equilibrium. The pressure of N2(g) is then 2.28 atm. Calculate KP for this reaction. (10) 3 O2(g) + 4 NH3(g) 6 H2O(g) + 2 N2(g)