DEHYDRATION OF 2-METHYLCYCLOHEXANOL
advertisement

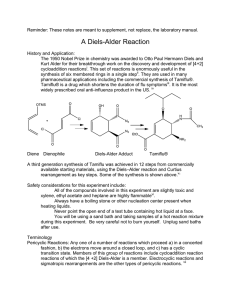
Chem 324 B. Terem DIELS-ALDER REACTION (in water) Diels-Alder reactions are very important tools in carbon-carbon bond formation. While most Diels-Alder reactions are carried out in inert hydrocarbon solvents at high temperatures, the current reaction is carried out in water, which accelerates the rate due to the hydrophobic effect in water. Using water as the solvent is also significant from an environmental point of view. O + N CH3 H2O O O anthracene-9-methanol N OH OH N-methylmaleimide CH3 O Experimental: SAFETY PRECAUTIONS: N-methylmaleimide is corrosive and should be handled with care. Wear gloves at all times for this experiment (in addition to all the other required safety precautions) Usually reactions of this type can be monitored by tlc to follow the formation of the product and the consumption of the starting materials (disappearance of the spots corresponding to the starting materials). However, we will only run tlc plates first to determine rf values for the starting materials and at the end of the reaction to determine the rf value of the product. Do not run any tlc plates before you start the Diels-Alder reaction. You can carry out the tlc analyses during the hour while the reaction mixture is refluxing (for time management purposes) Refer to last semester’s handout for tlc directions Prepare a tlc bath containing hexane/ethylacetate (1/1). Make sure to keep the tlc bath covered since evaporation might alter the ratio of the solvent mixture. Save a few crystals each of anthracene-9-methanol and N-methylmaleimide in separate small vials. Chem 324 B. Terem Dissolve the crystals in 10 drops (approximately) of acetone and spot each of them on the same tlc plate side by side. Develop the tlc plate and determine rf values for each of the reactants. Carry out a similar tlc analysis at the end of the reaction with the filtered solid product. Compare the two tlc plates (the present one and the one with the starting materials). Record your observations. Procedure: Place 0.10 g anthracene-9-methanol and 50 mL of water in a 100 mL round bottom flask equipped with a stir bar. Add 0.16 g N-methylmaleimide to the reaction flask. Fit the flask with a water cooled condenser and heat the reaction in a sand bath placed on a stirrer-hot plate until water starts to reflux. Keep the reaction refluxing for one hour with stirring. At the end of one hour remove the flask from the sand bath and allow it to cool to room temperature. Chill the flask in an ice bath and collect the crystals by filtration. Place your crystals in a small beaker and place the beaker into the oven. The product will be dry enough to weigh within 30 minutes. Weigh your product and calculate the yield. Concepts to think about (please write a paragraph or two in your notebook as part of the conclusion to your experiment): There are several unique features of this reaction such as “green chemistry”, “atom yield/economy”, and “hydrophobic effect” as it relates to the reaction. You may wish to read about hydrophobic effect in a general chemistry book to refresh your memory. Other sources about hydrophobic effects related to Diels-Alder Reactions can be found in publications by Breslow et al. some of which are listed below: Journal of American Chemical Society, 1991, 113, 4340-1. Journal of American Chemical Society, 1995, 117, 9923-4. Pure and Applied Chemistry, 1998, 70, 1933-8. (You are not expected to read these publications unless you wish to; however, this would be a good starting point to see how research is reported as a scientific publication)