Homework 1.2 - Clydebank High School
advertisement
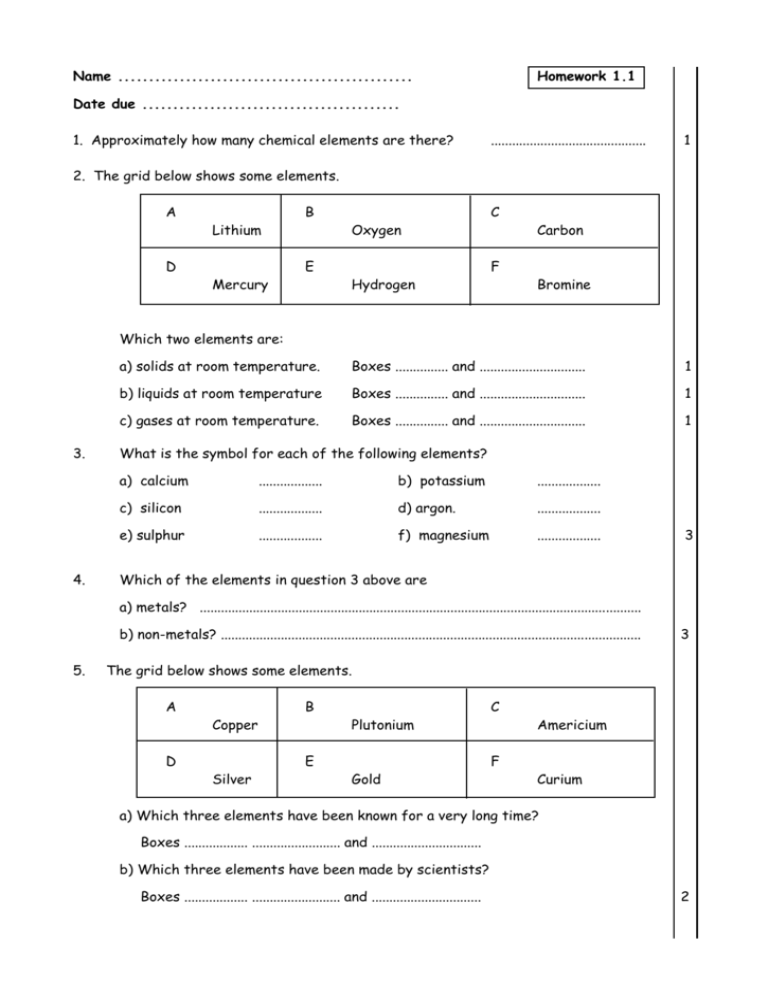
Name ................................................ Homework 1.1 Date due .......................................... 1. Approximately how many chemical elements are there? ............................................ 1 2. The grid below shows some elements. A D Lithium Mercury B E Oxygen Hydrogen C F Carbon Bromine Which two elements are: 3. 4. a) solids at room temperature. Boxes ............... and .............................. 1 b) liquids at room temperature Boxes ............... and .............................. 1 c) gases at room temperature. Boxes ............... and .............................. 1 What is the symbol for each of the following elements? a) calcium .................. b) potassium .................. c) silicon .................. d) argon. .................. e) sulphur .................. f) magnesium .................. 3 Which of the elements in question 3 above are a) metals? ............................................................................................................................. b) non-metals? ....................................................................................................................... 5. 3 The grid below shows some elements. A D Copper Silver B E Plutonium Gold C F Americium Curium a) Which three elements have been known for a very long time? Boxes .................. ......................... and ............................... b) Which three elements have been made by scientists? Boxes .................. ......................... and ............................... 2 Homework 1.1 6. What name is given to a substance which is formed when two or more different elements are joined together? ................................................................................................................................................ 1 7. Name the elements which were named after a) Marie Curie ................................................ b) Dimitri Mendeleev ................................................ 2 8. The grid shows some hazard symbols A B C D X Which box shows the symbol for a) a harmful substance? Box .................................. b) a radioactive substance? Box .................................. c) a corrosive substance? Box .................................. 3 9. Use the date of discovery table in the data booklet to answer the following. a) When was hydrogen discovered? date ................................. b) Which two elements were discovered in 1774? ............................. and ............................ 1 1 Name ................................................ Homework 1.2 Date due .......................................... 1. The air is a mixture of gases. Give the approximate percentage of each of the gases shown below in the air. Oxygen ................................ % of the air. Nitrogen .............................. % of the air. 2 2. The results of some chemical tests carried out on three gases, A, B and Cs, are shown in the table below. Effect of gas on Gas Glowing splint Limewater A splint goes out turns chalky B splint goes out no effect C splint relights no effect Which gas (A, B or C) is a) Oxygen Gas ........................... 1 b) Carbon dioxide Gas ........................... 1 3. Use you data book to complete the table below which gives some information about different metal elements. Metal Date of discovery Melting point (oC) Density (units) Aluminium Use 2.70 prehistoric water pipes 3 4. The grid shows the names of some commonly uses gases. A nitrogen B chlorine C oxygen D argon Which box shows the name of the gas used for a) filling lights bulbs. b) killing bacteria. 2 Homework 1.2 6. Use the tables of melting and boiling points on page 4 of the data book to answer the following a) At what temperature will lithium iodide become a liquid? .............................oC 1 b) What state (solid, liquid or gas) will phenol be in at a temperature of 100oC? .............................. 1 c) Which two molecular compounds will be gases at room temperature? ................................................... and ........................................................ 1 7. Use the table of solubilities on page 4 of the data booklet to give the solubility of the compounds below. a) iron nitrate ............................................................ b) calcium sulphate ............................................................ c) magnesium phosphate ............................................................ 3 8. The table below gives the melting points of four different elements. Use this information to construct a bar chart. Element Iodine Lithium lead Tin Melting point (oC) 114 181 328 232 melting point (oC) 3 Homework 1.2 9. Part of a pupil’s PPA write up is shown below. What was the aim of the experiment? To find out how the temperature of water affects the dissolving speed of sugar in water. Procedure :- a) What factor would the pupil have changed in her investigation? ........................................ ............................................................................................................................................................... 1 b) When this investigation is carried out what would be measured that told you how quickly the sugar crystals dissolved in water? ............................................................................................................................................................... ............................................................................................................................................................... 1 Name ................................................ Homework 1.3 Date due .......................................... 1. Which of the following statements is true (You should make use of the table of solubilities on page 4 of the data booklet). A B C D All sulphates are very soluble in water Copper sulphate is less soluble than calcium sulphate All nitrates are very soluble in water Most carbonates are very soluble in water Underline the correct statement. 1 2. The table shows the mass of sodium phosphate which can be dissolved in 100 g of water at different temperatures. Temperature in oC 10 30 50 70 Mass of copper sulphate in grams 13 18 25 37 (a) Use the information in the table to plot a line graph. Use an appropriate scale to fill most of the line graph paper. (b) Predict the mass of sodium phosphate which could be dissolved in 100g of water at 80oC. ..................................... 3 1 Homework 1.3 3. Name the elements present in the following compounds. a) Potassium bromide ........................................................................................................... 1 b) Lead carbonate ........................................................................................................... 2 4. The grid below gives the names of some substances. A zinc phosphate B lead C sodium fluoride D air Which box shows a) an element? Box letter ....... b) a compound containing three elements one of which is oxygen ? Box letter ....... c) a mixture? Box letter ....... d) a compound which contains only two elements? Box letter ....... 4 5. Which of the following are chemical reactions? Underline your answers. a match burning frying an egg ice melting dissolving sugar in water paint drying super glue setting 3 6. When a drop of water is added to a mixture of silvery coloured aluminium metal powder and grey-black iodine crystals a chemical reaction starts. Purple fumes are given off and the mixture catches fire. At the end of the reaction a light grey solid remains. Give three pieces of evidence which tells us that a chemical reaction has taken place. 1. ....................................................................................................................................................... 2. ....................................................................................................................................................... 3. ....................................................................................................................................................... 3 7. Explain the difference between concentrated and dilute orange juice. Use the words ‘solvent”, “solute” and “solution” in your answer. ....................................................................................................................................................... ....................................................................................................................................................... ....................................................................................................................................................... 2 Name ................................................ Homework 1.4 Date due .......................................... 1. A pupil added lumps of zinc metal to a dilute solution of hydrochloric acid at room temperature. The reaction between the zinc and the acid was very slow. Give three ways of increasing the speed of the reaction. 1. ....................................................................................................................................................... 2. ....................................................................................................................................................... 3. ....................................................................................................................................................... 3 2 .Magnesium reacts with hydrochloric acid to produce hydrogen gas. In the experiment shown below the volume of hydrogen gas collected in the syringe was recorded at equal time intervals. gas syringe magnesium Time (seconds) 0 20 40 60 80 100 hydrochloric acid Volume of gas (cm3) 0 16 24 30 33 35 a) What was the time interval between each measurement? ................................ s b) Draw a line graph of the results in the table. 1 3 Homework 1.4 2. c) Use your graph to give the volume of gas that was produced after 50 seconds. ...............cm3 1 3. In which of the following would the sugar dissolve most quickly? Underline the correct answer. A. lump sugar in water at 20oC B. lump sugar in water at 30oC C. powdered sugar in water at 20oC D. powdered sugar in water at 30oC 1 4. a) What does a catalyst do to a reaction? ............................................................................. 1 b) At the start of a reaction 2g of a catalyst was added. What mass of catalyst would be present at the end of the reaction? ............... g 1 c) What name is given to a biological catalyst? ........................................ 1 d) Name the metal used as the catalyst in a catalytic converter fitted to a car exhaust. ......................................... 1 5. Name the gas used to make drinks such as lemonade fizzy. ............................................... 1 6. Why is sodium fluoride added to drinking water and toothpastes? ................................... ............................................................................................................................................................... 1 7. Write word equations for each of the following. a) Magnesium reacting with oxygen to form magnesium oxide. .............................................................................................................................................................. 1 b) Sodium chloride and water are formed when sodium hydroxide and hydrochloric acid react together. .............................................................................................................................................................. 1 8. The word equation below shows what happens when methane gas is burned. Methane + oxygen carbon dioxide + water a) Name the two products in this reaction. ................................................................................ 1 b) Name the two reactants in this reaction ................................................................................ 1 c) Which of the substances shown in the word equation is an element? ............................. 1 Name ................................................ Homework 1.5 Date due .......................................... 1. What name is given to the tiny particles which make up elements? .................................... 1 2. Using your data booklet identify a) the element with an atomic number of 12. ............................................................ 1 b) the atomic number of the element carbon. ........................................................... 1 3. Use the models shown below to write the formula for each of the substances. a) nitrogen atom b) c) hydrogen atom Formula = ......................... 1 chlorine atom Formula = ........................ 1 Carbon atom Hydrogen atom Oxygen atom Formula = .......................... 1 4. Write the formula of each of the following compounds. a) H H H H C C C H H H H Formula = .................................. 1 Formula = ...................................... 1 Formula = ........................................ 1 b) O H H C C H C H H H c) O H C H H H C C H O C H H H Homework 1.5 5. Write the formula for each of the following compounds. a) Sulphur trioxide Formula = ................................ b) Carbon tetrachloride Formula = ................................ c) Dinitrogen monoxide Formula = ............................... 3 6. Complete the following sentences by scoring out the incorrect words. The bonds that hold the atoms together inside a molecules are strong/weak. The bonds between the molecules are strong/weak. Substances which are made up of molecules have low/high melting points. 3 7. Solid ionic compounds such as sodium chloride, do not conduct electricity when solid but they do conduct when dissolved in water. a) Explain why the solid ionic compound does not conduct electricity. …………………………………………………………………………………………………………………………………………………. 1 b) Explain why sodium chloride solution does conduct electricity. …………………………………………………………………………………………………………………………………………………. 1 8. The diagrams below represent different elements and compounds. A B C Which diagram represents a) an ionic compound? Box ………………… 1 b) an element Box ……………….. 1 Box ……………….. 1 c) a molecular compound Name ................................................ Homework 1.6 Date due .......................................... 1. What is the pH value of a neutral solution? pH ……………….. 1 2. The pH values of 4 solutions is shown in the table below. Solution W X Y Z pH value 9 2 5 12 a) Which solution is the most acidic? Solution ………… 1 b) Which two solutions are alkaline? Solutions …………… and …………… 1 3. Complete the following sentences by scoring out the incorrect words. When an alkali solution is diluted by adding water to it the pH value increases/decreases and the alkalinity increases/decreases. 2 4. a) Describe how Universal Indicator solution can be used to test the pH value of vinegar. ………………………………………………………………………………………………………………………………………………………….. ………………………………………………………………………………………………………………………………………………………….. b) Instead of using Universal Indicator solution, what else could be used to find the pH of a solution? …………………………………………………………………………………………………………………………………………………………. 2 1 5. Arrange the following household chemicals in the correct column in the table. baking soda; soda water; Acids vinegar; oven cleaner; lemonade; Alkalis 2 Homework 1.6 6. The bar chart gives the pH values of some chemicals labelled A to E. 14 12 pH 10 8 6 4 2 0 A B C Chemical D E a) Which two chemicals are acidic? ……………………. and ………………… 1 b) Which chemical is most alkaline? ……………….. 1 c) Which chemical could have been water? ……………….. 1 d) What colour would you expect to see when chemical E is tested with Universal Indicator solution? …………………. 1 7. a) Name two common laboratory acids. ……………………………………………………………………………………………………………………………………………….. 2 b) Name two common laboratory alkalis. ……………………………………………………………………………………………………………………………………………….. 2 8. A pupil recorded the pH of a number of different solutions. Her results are shown below. bicarbonate of soda = 9; detergent = 8; lemonade = 5; milk = 8; soap = 9; orange juice = 4; tap water = 7 Present these results in the form of a table with two headings. 2 Name ................................................ Homework 1.7 Date due .......................................... 1. Complete the word equation for a neutralisation reaction. Acid + Alkali ……………………….. + water 1 2. Complete the following sentences by scoring out the incorrect words. When an acid is neutralised by adding an alkali to it the pH of the acid rises/falls towards 7. When an alkali is neutralised by adding an acid to it the pH of the alkali rises/falls towards 7. 2 3. Complete the following sentences by filling in the missing words. Sodium chloride is a salt made when sodium hydroxide is neutralised using ……………………………………….. acid. Calcium sulphate is a salt made by reacting …………………………. hydroxide with sulphuric acid. When potassium hydroxide reacts with nitric acid the salt …………………………………….. …………………………………. is formed. 3 4. Copper carbonate can be used to neutralise sulphuric acid. a) Name the elements present in copper carbonate. …………………………………………………………………………………………………………………………………………………… 1 b) Name the gas given off when copper carbonate reacts with sulphuric acid. ……………………………………………………………………………………………………………………………………………………. 1 c) Name the salt formed when copper carbonate reacts with hydrochloric acid. ………………………………………………………………………………………………………………………………………………… 1 d) In this reaction an indicator is not needed to show when all of the acid has been neutralised. How would you know that all the acid has been neutralised? ………………………………………………………………………………………………………………………………………………. 1 e) How is any excess copper carbonate powder removed from the salt solution? …………………………………………………………………………………………………………………………………………….. 1 Homework 1.7 5. What do farmers add to an acidic soil to neutralise the acid in the soil? …………………………………………………………………………………………………………………………………………………………. 1 6. Name two non-metal oxide gases which dissolve in water to form acid rain. …………………………………………………………………….. and …………………………………………………………………………… 1 7. Nitrogen and oxygen in the air do not normally react. However, inside a car engine these two gases combine to form nitrogen dioxide. a) Write a word equation for the reaction. ………………………………………………………………………………………………………………………………………………………….. 1 b) Why are nitrogen and oxygen able to react inside a car engine when they will not react in the atmosphere? …………………………………………………………………………………………………………………………………………………….. 1 8. Give two examples of the damage that acid rain causes. 1 …………………………………………………………………………………………………………………………………………………………… 1 2 …………………………………………………………………………………………………………………………………………………………… 1 9. An indigestion table contains the following chemicals. Calcium carbonate Magnesium carbonate Sodium bicarbonate Magnesium silicate 150 mg 80 mg 120 mg 90 mg Present this information as a bar graph. 3