File
advertisement

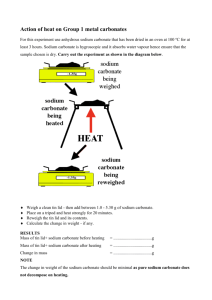
Leaving Certificate 2014/15 Mandatory Experiments Below is a suggested breakdown of the 28 mandatory experiments to answer questions in Section A. Parts of these experiments may occur randomly in Section B also. Q.1 Q.1 Q.1 Q.1 Q.1 Q.1 Q.1 Q.1 Q.1 Q.1 Q.2 Q.2 Q.2 Q.2 Q.2 Q.2 Q.2 Q.2 Q.3 Q.3 Preparation of a standard solution of sodium carbonate Standardisation of a hydrochloric acid solution Hydrochloric acid / Sodium hydroxide titration and use of this reaction to make salt NaCl Determination of the concentration of ethanoic acid in vinegar Determination percentage water of crystallisation in a sample of hydrated sodium carbonate A potassium manganate (VII) / ammonium iron(II) sulphate titration Determination of the amount of iron in an iron tablet An iodine \ thiosulphate titration Determination of the percentage of hypochlorite in bleach Estimation of total hardness using ethylenediaminetetraacetic acid. [edta] Estimation of dissolved oxygen by redox titration Preparation and properties of ethene Preparation and properties of ethyne A. Extraction of clove oil from cloves [or similar] by steam distillation B. To isolate clove oil (eugenol) from an emulsion of clove oil and water by liquid-liquid extraction using cyclohexane. Preparation of soap Reactions of Ethanal Reactions of Ethanoic Acid To oxidise phenylmethanol (benzyl alcohol) to benzoic acid with potassium permanganate solution in alkaline conditions. Recrystallisation of benzoic acid and determination of its melting point Q.3 Q.3 Q.3 Flame tests To test for anions: chloride, carbonate, hydrogen carbonate, nitrate, sulphate, phosphate, and sulphite. To find the relative molecular mass of a volatile liquid Redox reactions of Group VII elements. Halogens as oxidising agents with bromides, iodides, Iron(II) and sulphites Displacement reactions of metals Monitoring the rate of production of oxygen from hydrogen peroxide using MnO2 Determination of the heat of reaction of hydrochloric acid with sodium hydroxide Studying the effects on the reaction rate of (i) concentration, (ii) temperature Q.3 Simple experiments to Illustrate Le Chatelier’s Principle *Q.3 To determine total suspended and dissolved solids in p.p.m. by filtration and evaporation, determine pH Q.3 Colorimetric expt to estimate the concentration of free chlorine in swimming pool or bleach Q.3 Q.3 M.Healy, PCH 2014/15