CONFORMATIONS OF ALKANES AND CYCLOALKANES
advertisement

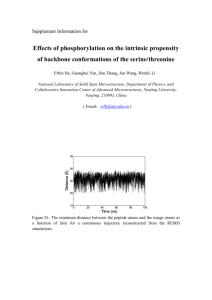
Honors Organic Chemistry CONFORMATIONS OF ALKANES AND CYCLOALKANES Purpose: The purpose of this activity is to explore the different conformations of simple alkane and cycloalkane molecules. Materials: Lab sheets, Model kits, Laptops with ChemSketch Safety: Do not throw or ingest atoms or bonds from the model kit. Discussion: Newman Projections The bonds between carbon atoms in alkanes have the ability to rotate freely around the sigma bond. This ability to rotate freely around bonds produces various conformations, or arrangements around the carbon-carbon single bond. For example, the carbon-carbon single bond in ethane rotates to form two basic conformations: H H H H H H H H H H Staggered conformation H H Eclipsed conformation These conformations can be viewed in a much easier fashion by looking down the carbon-carbon single bond. Such views are called Newman projections, and are exemplified below: H H H H ) H H H H H H H Back carbon (circle) H Front carbon (intersection) The “front” carbon is represented by the intersection of the bonds to its three attached hydrogens. The bonds from the remaining three hydrogens connect to the circle, which represents the “back” carbon. Propane and butane can also be easily depicted using Newman projections. Each conformation of each molecule of ethane, propane, and butane has different amounts of potential energy, depending on how the atoms within the molecules are arranged. A molecule that has “interference” between different atoms has a higher potential energy than one whose atoms are as far apart as possible. We can use Newman projections to see each different conformation of ethane, propane, and butane, then assess whether the conformation is stable or not. Procedure 1. Using Newman projections, draw two different conformations of ethane. Label each conformation as eclipsed or staggered. Construct ethane with the model kit to help see the different conformations of ethane. Draw ethane on ChemSketch (draw all hydrogens, too). Using your knowledge of electron-pair repulsion and how it contributes to strain, determine which conformations of ethane have the highest and lowest potential energy. Diagram Conformation Potential Energy 2. How would the Newman projection change for propane? Build the model, draw the Newman projections below and draw the molecule on ChemSketch. Label the conformations and compare their potential energies. Diagram Conformation Potential Energy 3. Draw the most stable and least stable conformations of butane. Build the model, draw the Newman projections below and draw the molecule on ChemSketch. Label the conformations and compare their potential energies. Diagram Conformation Potential Energy Cahn-Ingold-Prelog R-S Notational System Another method of studying 3D conformations was developed for substituted alkanes in 1956. This system, called the Cahn-Ingold-Prelog R-S Notational System, uses the letters R and S to describe the arrangement of different atoms around a carbon. For example, bromochlorofluoromethane has four different atoms bonded the central carbon. Three dimensionally, these atoms can be arranged differently. Br Cl Br H or Cl F F A B H or A B These molecules are actually mirror images of each other. Rotate molecule B 180°. Cl Cl Br H or Br H or F F A B A B If superimposed onto each other, molecules A and B do not match. Mirror–image representations of molecules that cannot be superimposed onto each other are called chiral molecules. They are also called enantiomers of each other. Mirror–image representations of molecules that can be superimposed onto each other are called achiral molecules. A tetrahedral carbon atom with four different substituents is called a stereogenic center. The Cahn-Ingold-Prelog R-S Notational System prioritizes the four different substituents on a stereogenic center according to atomic mass. For example, bromochlorofluoromethane is prioritized according to decreasing atomic mass as Br, Cl, F, and H. To assign R or S, arrange the stereogenic center with the lowest priority atom pointed away from you. Cl Cl Br H Molecule A: → Br F F Priority ranking appears counterclockwise, therefore assigned as the S conformation. (S) – bromochlorofluoromethane Br Br Molecule B: Cl H F → Cl F Priority ranking appears clockwise, therefore assigned as the R conformation. (R) - bromochlorofluoromethane The usual physical properties like density, melting point, and boiling point are identical for both enantiomers of chiral compounds. Other properties that may differ are those affected by the arrangement of atoms in space. For example, the enantiomers (R)– carvone and (S)–carvone differ in their odor. (R)–carvone comes from spearmint oil while (S)–carvone comes from caraway seed oil. The difference in odor between the two enantiomers results from how the spatial arrangement of the atoms interacts toward receptor sites in the nose! Procedure 4. Construct the R and S conformations of bromochlorofluoroiodomethane (use orange for bromine, green for chlorine, blue for fluorine and purple for iodine) and 1-aminoethanol (CH3CH(NH2)OH). After identifying the stereogenic center, draw their 3D Kekulé representations. Draw and name these compounds on ChemSketch. Conformation Bromochlorofluoroiodomethane 1-aminoethanol R S 5. Up until the late 1950s, most chiral over-the-counter or prescription drugs were unknowingly sold as 50/50 mixtures of their two different enantiometric forms (called a racemic mixture). What problems may arise from this lack of knowledge of chirality in organic molecules such as drugs? Look up thalidomide for a good example… where is the stereogenic center? O N O O H N O Conformations of Cycloalkanes Cycloalkanes are usually viewed as two-dimensional geometric shapes, but actually exist as three-dimensional molecules. Carbon compounds tend to form stable tetrahedral shapes with bond angles of 109.5˚, but smaller cycloalkanes differ considerably from tetrahedral shape. This difference between actual shape and tetrahedral shape causes a stability problem called ring strain. As the number of carbons increases, there is a decrease in the amount of ring strain within the molecule. Substituted cycloalkanes have another aspect to consider when determining strainless conformations. Eclipsing hydrogens cause problems in the lower cycloalkanes, but again, as ring size increases, the hydrogens adopt staggered conformations, further reducing the amount of ring strain. Alkyl substituent groups produce more strain on the ring, causing the molecule to adopt new, more stable conformations. Procedure 1. Construct cyclopropane with the model kit (use long bonds for C–C bonds). Draw cyclopropane on ChemSketch. Look at it through the 3D viewer. a. Is cyclopropane planar or nonplanar? How does this affect its stability? b. How do the hydrogen atom arrangements affect its stability? 2. Construct cyclobutane with the model kit. Draw cyclobutane on ChemSketch. Look at it through the 3D viewer. a. Is cyclobutane planar or nonplanar? How does this affect its stability? b. How do the hydrogen atom arrangements affect its stability? c. Which is more reactive; cyclopropane or cyclobutane? Explain. 3. Construct cyclopentane with the model kit. Draw cyclopentane on ChemSketch. Look at it through the 3D viewer. a. Is cyclopentane planar or nonplanar? How does this affect its stability? b. How do the hydrogen atom arrangements affect its stability? c. Which is more reactive, cyclobutane or cyclopentane? Explain. 4. Construct cyclohexane with the model kit. Find the “boat” conformation and the “chair” conformation. Draw cyclohexane on ChemSketch. Look at it through the 3D viewer. a. Is cyclohexane planar or nonplanar? Which conformation does the molecule form in ChemSketch? How does this affect its stability? b. How do the hydrogen atom arrangements affect its stability? c. Which conformation of cyclohexane is more stable? Explain.