Combating Inheritable heart disease: Functional and Molecular
advertisement

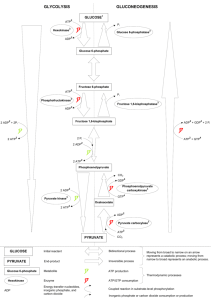
Title of project: Combating Inheritable heart disease: Functional and Molecular characterisation of Mitochondrial ADP/ATP carrier Director of Studies: Dr Vincent Postis Second Supervisor: Prof Guy Lauquin Overview of project Mitochondrial adenine nucleotide carriers are polytopic proteins located in the inner mitochondrial membrane. They are essential for life as they are one of the main sources of ATP supply for the cell thanks to their ability to export matrix ATP, synthesised by the respiratory chain, and import cytosolic ADP, thus providing new substrate for the ATP synthase. Three tissue specific isoforms exist in humans. Point mutations in the human cardiac isoform (hANC1) have been shown to be involved in two main pathologies: myopathies and autosomal dominant progressive external ophtalmoplegia (adPEO). The first affect muscles and the second is clinically characterized by exercise intolerance, ptosis and muscle weakness. The mechanisms underlying the mitochondrial pathologies caused by hANC1 mutations remain largely poorly understood. In the light of extensive biochemical studies, it is admitted that these transporters switch between at least four conformations (ADP binding/release and ATP binding/release), two of which can be frozen by the use of specific inhibitors called carboxyatractylate (CATR) and bongkrekic acid (BA). In 2003, the molecular structure of the bovine homologue of hAnc1 has been solved in a CATR conformation, allowing to understand one of the conformations the transporter adopt during the ADP/ATP exchange. The complete cycle, however, has not been yet understood due in part to the inability to isolate a BAinhibited conformation. In addition, although more than 20 years of biochemical data tend to indicate that these carriers function as dimers, the crystal structure only showed a monomer and opened an endless debate. The aim of the project is to isolate and structurally characterise the adenine nucleotide translocators through the solving of their structure in a BA conformation as well as isolating the transporter in his native lipidic environment. Unlike conventional techniques using detergents which have failed to produce crystal of this transporter in its 'BA conformation', this study will use a cutting edge methodology which take advantage of the SMALP (styrene maleimic acid lipoparticles) technique. To maximise the applicant chance of success, the human ANC1 protein will be studied together with the yeast homologue ANC2. Both isoforms genes are readily available from Dr Postis previous work (de Marcos Lousa et al., 2003; Postis et al., 2004). These will need to be cloned in an adequate vector for expression in mammalian cells such as HEK cells, which have been demonstrated to be a reliable system for membrane protein expression. Molecular studies using SMALP technology has been pioneered by Dr Postis (Postis et al., 2015). Hence this project will combine basic molecular biology and biochemistry, ideal techniques to be learned by a PhD student for future employability. Link to Faculty Research Themes School of Rehabilitation and Health Sciences 5A (Biological Sciences) Outline of project including proposed timescales This project will give the opportunity to the PhD student's to develop skills in a cutting edge methodology highly looked at by the pharmaceutical industry. Finally, the structure resolution will be solved in collaboration with prof. Goldman, which is a renowned scientist in the crystallography field. This will allow the PhD student to create a network for collaborators for future job/grant applications. This work outcome will be disseminated through publication in high impact journals, such as JBC (impact factor 4.6) and JMB (impact factor 3.9). The results obtained during the first year of this program will be used as support for a BBSRC New Investigator Scheme application for Dr Postis Further information To apply you must be eligible for NHS Continuing Professional Development (CPD) funding and have the support of your line manager in writing. General enquiries should be directed by email to the Faculty Research Director r.hogston@leedsbeckett.ac.uk to discuss the project further please contact the Director of Studies V.L.Postis@leedsbeckett.ac.uk Applications should be made on line here http://www.leedsbeckett.ac.uk/research/research-degrees/research-studentships-andfees-only-bursaries/