File
advertisement

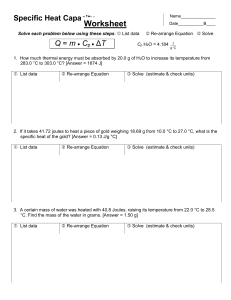
Name_______________ Specific Heat Capacity Date___________B____ Another unique property of water is that it has a very high _________________________________. This means that water can ____________ and ______________ large amounts of _________ as it is heated or cooled. However, these large changes in energy only result in ___________ changes in _________________. In other words, water resists changes in temperature. This is because in before you can make the molecules move ____________ the hydrogen bonds between them have to be _____________. Therefore, when you heat water most of the energy actually goes into breaking the hydrogen bonds. Water’s high specific heat helps to stabilize your body’s __________________ and the Earth’s different ____________ within a suitable range for life. Specific heat is defined as: Equation Variable Units A little chemistry algebra! Solve for m Solve for Cp Solve for Δ Example Problem: A sample of water releases 2510.4 J of energy as its temperature goes from 26°C to 23°C. What is the mass of this sample of water? List data Re-arrange Equation Solve (estimate & check units) Specific Heat Capacity ClassworkHW Name_______________ Date___________B____ 1) How much energy is needed to raise the temperature of a 55 g sample of aluminum from 22.4°C to 94.6°C, if Cp of Al = 0.897 J ? [Answer = 3,562 J] g C List data Re-arrange Equation Solve (estimate & check units) 2) It takes 142.2 J to raise the temperature of 34.0 g of ammonia, NH3 (g), from 23.0°C to 25.0°C. Calculate the specific heat (Cp) of ammonia. [Answer = 2.1 J ] g C List data Re-arrange Equation Solve (estimate & check units) 3) 50.0 grams of water heats up from 24°C to 29°C. How much energy is absorbed by the water? List data Re-arrange Equation Solve (estimate & check units) 4) 100.0 g H2O loses 5020.8 J of energy as it cools. What is the change in temperature? [Ans. 12°C] List data Re-arrange Equation Solve (estimate & check units)