Physical Properties (2-2)
advertisement

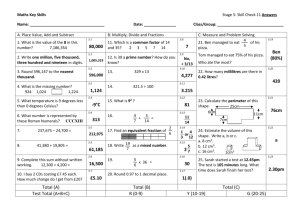
The Properties of Matter Chapter 2 What is matter? (2-1) Matter is anything that has mass and volume. *Mass and weight are different!* 1 Physical Properties (2-2) A physical property of matter can be observed or measured without changing the matter’s identity. examples: thermal conductivity (is it a conductor or insulator) (solid, liquid, gas, plasma) state density solubility (does it dissolve) ductility (draw out into wire) malleability (pound into shapes) odor, color, melting point, boiling point 2 Density is the amount of matter in a given space, or volume. Density = Mass / Volume D=M V Units for density are derived from mass /volume: g/cm3 g/mL kg/m3 3 Practice: 1. Find the density of a substance that has a mass of 3 45 kg and a volume of 43m . D = M/V D = 45 kg / 43 m3 D = 1.05 kg/ m3 2. What is the density of an object whose mass is 25g and whose volume is 10 cm3? D = M/V D = 25 g / 10 cm3 D = 2.5 g/ cm3 4 A physical change affects one or more physical properties of a substance without changing the identity of the substance. *the identity of the substance does not change! melting, freezing, boiling, cutting, bending, dissolving (see poster) 5 Chemical Properties (2-3) A chemical property describes a substance’s ability to participate in chemical reactions. (or, ability to change into new matter) examples: flammability (combustibility) acidity basicity (alkaline) reactivity 6 A chemical change is when substances are changed into new substances with new properties. *identity of matter is changed! examples: soured milk, rusting nail, burning wood, baking cake, digesting food (see poster) (transparency) 7 Evidence of a chemical reaction includes the following signs: producing a gas (bubbling) change in color or odor change in energy (gives off heat or takes in heat) produces sound or light 8