Density Lecture
advertisement
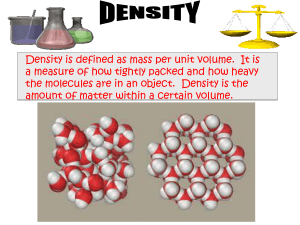
Density What weighs more… A pound of lead or a pound of feathers? There is a relationship between an objects mass and its volume. Density: the ratio of mass of an object to its volume. Density = Mass Volume A 10 cm3 piece of lead has a mass of 114 g. What is its density? Density = Mass Volume Density depends on the composition of the substance, not on its size Calculating Density. A copper penny has a mass of 3.1 g and a volume of .35 cm3. What is the density of copper? A bar of silver has a mass of 68.0 g and a volume of 6.48 cm3. What is the density of Au. Using Density to Calculate Volume What is the volume of a pure silver coin that has a mass of 14 g? (density Au = 10.5 g/cm3)