Notes 6
advertisement

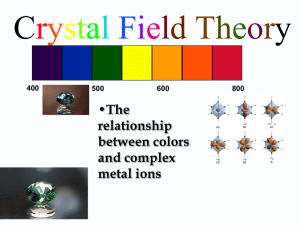
Notes 6 Complex Ions are characterized according to 1. the central metal cation 2. the _____________________ and/or ___________ to which the cation is complexed (bonded) called the ligands. 3. The number of ___________ ____________ to the central metal cation is referred to as its coordination number. 1. Complex ions formed commonly by transition metals, especially on right side of the transition series. Some non-transition metal ions such as Al, Sn, and Pb, ions also form stable complex ions. 2. Ligands can be neutral or charged; if charged they contribute to the overall charge. If they are not charged, like ammonia or water, then they don’t contribute. Some ligands have more than one atom with an unshared pair of electrons. This is known as a chelate. More than one bond forms with a central atom if the chelate forms a five or six membered ring when the two bonds form. Smaller and larger rings are less stable: Examples: Oxalate ion, C2O42 - Hydrazine Ethylenediamine molecule has two lone pairs but does not form a chelate, why? Charge of the complex = charge on central _________ _________ + charges of _________ 3. The most common coordination number is ______. 4 is a bit less common, and 2 is almost exclusively associated with Cu+ , Ag+ , Au+ . Associated with these different coordination numbers are different geometries “molecular geometries” Coordination number Geometry 2 4 square 4 6 Many cations have more than one coordination number, but a few only have one cation. These include Pd2 + , Pt2 + , with coordination number of 4 and a square planar geometry Cd2 + and Zn2 + with a coordination number of 4 and a tetrahedral geometry Co3 + , Cr3 + , Fe2 + , and Fe3 + with a coordination number of 6 and an octahedral geometry Others such as Cu+ , Cu2 + , Ni2 + can have more than one coordination # See Table . Examples Write the formulas (including charges) of all complex ions formed by cobalt(III) with the hydroxide ion and/or ethylenediamine (en) molecules as ligands. Complex Ions are often colorful compounds. What is it about these materials that allow them to be colored? Must consider the ______________ _______________ of the central metal cation and how the ligands interact with the orbitals occupied by the electrons. Which orbitals are populated by the valence electrons in a transition metal ion.? What are the shapes of these orbitals? How do the ligands interact with these orbitals? Octahedral Complexes The six ligands bound in an octahedral complex change the energies of the electrons in the d orbitals splitting into two groups of different energy. 1. A higher energy pair, the dx2-y2 and dz2 orbitals. 2. A lower energy trio, the dxy, dyz, and _________ orbitals. The energy difference between the two is called the crystal __________ _________ energy. The ligands in an octahedral complex interact more strongly with the d orbitals that have their maximum electron density along the x, y, and z axis. (So the dx2-y2 and dz2 orbitals. The result is that the energy of the d orbitals is split. into two types: 1. a low-spin complex containing the minimum number of unpaired electrons, formed when the splitting energy is rather large so that the electrons pair up before going into the higher level. 2. a high spin complex containing the maximum number of unpaired electrons because the energy difference is small enough the electrons will go into the higher energy level before spin pairing in the lower energy level. See examples of Crystal Field Splitting Color The color of a compound or complex ion is the result of what is left over when the absorbed color of light is subtracted from the visible spectrum. Essentially the color of a complex is the complimentary color, (opposite color on the color wheel), to the color of the light absorbed. The energy of the light absorbed is the energy difference between the higher energy d orbitals and the lower energy d orbitals described in crystal field splitting. The crystal field splitting energy, o, depends on which ligands are present on the central metal ion. A large splitting energy is called strong field and a small splitting is called weak field. Weak field is small energy splitting so long wavelength light is absorbed and strong field is large energy splitting so short wavelength light according to o = Ephoton = hc/ CN > NO2 > en > NH3 > NCS- > H2O > F- > Clstrong field weak field See table 15.3 How does this change for a tetrahedral complex? Intermolecular forces Types: Dipole-Dipole Interaction Ion Dipole Interaction Ion-Induced Dipole Interaction London or Dispersion Interactions Hydrogen Bonding See Table of interaction p. 502 Repulsive and Total Interactions