CHM 234: Worksheet #1
advertisement
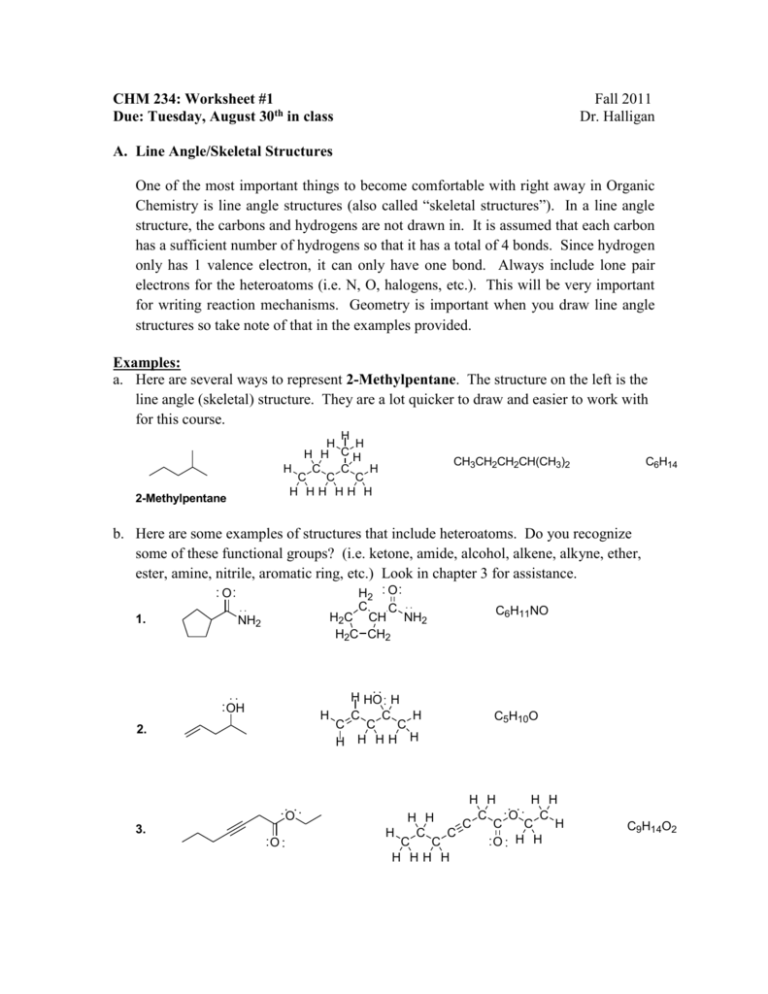
CHM 234: Worksheet #1 Due: Tuesday, August 30th in class Fall 2011 Dr. Halligan A. Line Angle/Skeletal Structures One of the most important things to become comfortable with right away in Organic Chemistry is line angle structures (also called “skeletal structures”). In a line angle structure, the carbons and hydrogens are not drawn in. It is assumed that each carbon has a sufficient number of hydrogens so that it has a total of 4 bonds. Since hydrogen only has 1 valence electron, it can only have one bond. Always include lone pair electrons for the heteroatoms (i.e. N, O, halogens, etc.). This will be very important for writing reaction mechanisms. Geometry is important when you draw line angle structures so take note of that in the examples provided. Examples: a. Here are several ways to represent 2-Methylpentane. The structure on the left is the line angle (skeletal) structure. They are a lot quicker to draw and easier to work with for this course. H H H C H H H H C C H C C C H HH HH H 2-Methylpentane CH3CH2CH2CH(CH3)2 C6H14 b. Here are some examples of structures that include heteroatoms. Do you recognize some of these functional groups? (i.e. ketone, amide, alcohol, alkene, alkyne, ether, ester, amine, nitrile, aromatic ring, etc.) Look in chapter 3 for assistance. H2 O C C H2C CH NH2 H2C CH2 O 1. NH2 H HO H H C C H C C C H H HH H OH 2. O 3. O C6H11NO C5H10O H H H H C O C H H C C C H H C C C C O H H H HH H C9H14O2 Problems: 1. Determine the molecular formula for the following structures (a-f). Write in the missing lone pairs of electrons. B. Degree of Unsaturation When determining a possible structure for a molecular formula, it is helpful to calculate the total number of rings + pi bonds (Degree of Unsaturation) that the structure will have. Example: Provide 3 possible skeletal structures for C7H10O. First calculate the Degree of Unsaturation: 2C + 2 - (H + X) + N U= 2 U = Degree of Unsaturation (total number of rings plus pi bonds) C = number of carbons H = number of hydrogens X = number of halogens (F, Cl, Br, I) N = number of nitrogens Note: The number of oxygen atoms does not appear in this equation. 2C + 2 - (H + X) + N 2(7) + 2 - (10 + 0) + 0 U= = 2 2 14 + 2 - (10 ) + 0 U 16 - 10 = = 2 2 6 = 3 = 2 Note: Be careful with the order of operations in this equation. Many people get the wrong answer. Second, draw a structure that has 7 carbons and a degree of unsaturation of 3 (total number of rings and pi bonds). Include the oxygen atom in this structure, keeping in mind that oxygen gets two bonds. If you do this, you will automatically have the right number of hydrogen atoms. O OH C7H10O C7H10O O C7H10O Problems: 2. Calculate the Degree of Unsaturation then provide two possible skeletal structures for each molecular formula. Draw in lone pairs of electrons where appropriate. a. C4H9N b. C5H10O2 c. C3H7NO d. C6H10Cl2 e. C11H11NO C: Formal Charges Formal charges are very important and must be included in our drawings. If you do not draw a formal charge when it is supposed to be drawn, then the drawing is incomplete (and wrong). When calculating formal charges, you need to first consider the number of valence electrons the atom is supposed to have (get this number from the periodic table). Second, look at the drawing and ask yourself how many electrons the atom actually possesses. Each atom owns its lone pair electrons and half of all the bonding electrons. Finally, determine the difference between these two numbers and see if you have an excess of electrons (negative charge), a deficiency of electrons (positive charge) or exactly the right number. Examples: Determine the formal charges for the following compounds. O a. b. c. N O Cl Br O d. e. f. N Answers: nc O a. b. Br d. N c. O Cl nc O e. f. N Problems: 3. For each of the compounds below determine if the oxygen or nitrogen atom in the molecule has a formal charge. If there is a charge, draw the charge near the atom and circle it. If the there is no formal charge, write “nc” near the molecule. O O N a. b. c. N N e. f N d. 4. In the next exercise you will encounter some atoms with a formal charge. Remember that carbon is in group 4 of the periodic table and is therefore usually seen with 4 bonds. If the carbon has a (+) charge, then it is because carbon only possesses 3 electrons so that means that there is one electron (and therefore one bond) missing. If the carbon has a (-) charge, then carbon must own 5 electrons which means it will have three bonds and one lone pair of electrons. A minus charge automatically signifies an extra lone pair of electrons. For each of the following problems, take a look at where the charges are located and draw in all missing lone pairs of electrons. The lone pairs of electrons will be on certain carbon, nitrogen and oxygen atoms. N O O O NH2 a. b. c. d. D: Resonance Structures In the next two questions, we will tackle resonance structures. A combination of resonance structures more accurately describes a particular molecule and helps us better understand the electronic nature of the compound of interest. Instead of just guessing what a resonance structure will look like, it is helpful to draw the arrows leading from one structure to the next. There are a few things you should keep in mind. There are two important “commandments” that you can never violate when pushing arrows. Example a. Thou shall not break a single bond. b. Thou shall not violate the octet rule. N N Problems: 5. Draw the curved arrows that get you from one resonance drawing to the next. When you draw arrows, you must begin at the source of electrons such as lone pairs and pi bonds. If you are using pi bond electrons, begin your arrow halfway across the bond. O O O O a. b. OH O O N N O O OH d. c. 6. In the following resonance structure problems, draw the resulting resonance structure after pushing the arrows shown. Be sure to include formal charges. Draw in all lone pairs of electrons. O N O b. a. O c. O O d.






