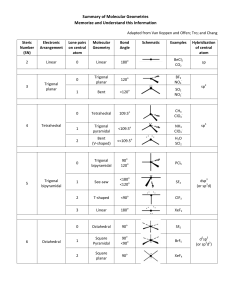
Molecular Geometry Worksheet - smhs
advertisement
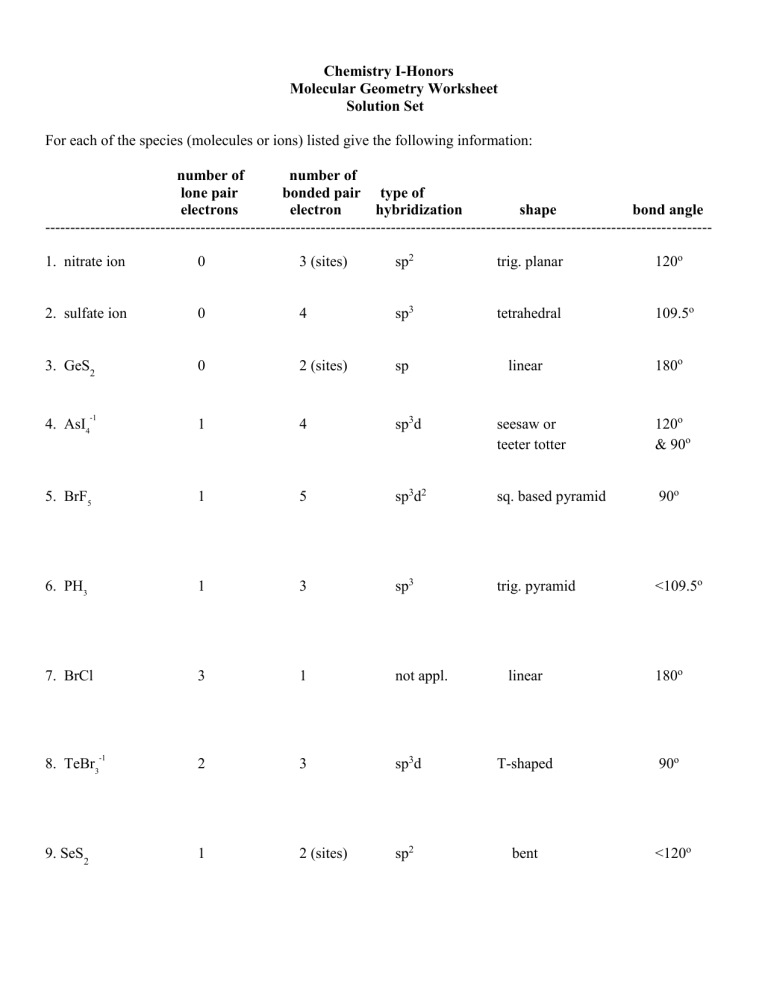
Chemistry I-Honors Molecular Geometry Worksheet Solution Set For each of the species (molecules or ions) listed give the following information: number of number of lone pair bonded pair type of electrons electron hybridization shape bond angle ------------------------------------------------------------------------------------------------------------------------------------1. nitrate ion 0 3 (sites) sp2 trig. planar 120o 2. sulfate ion 0 4 sp3 tetrahedral 109.5o 3. GeS2 0 2 (sites) sp linear -1 1 4 sp3d seesaw or teeter totter 120o & 90o 5. BrF5 1 5 sp3d2 sq. based pyramid 90o 6. PH3 1 3 sp3 trig. pyramid <109.5o 7. BrCl 3 1 not appl. 2 3 1 2 (sites) 4. AsI4 8. TeBr3 9. SeS2 -1 180o linear 180o sp3d T-shaped 90o sp2 bent <120o number of number of lone pair bonded pair type of electrons electron hybridization shape bond angle -------------------------------------------------------------------------------------------------------------------------------------1 3 2 sp3d linear 180o 0 6 sp3d2 octahedral 90o 1 3 sp3 trig. pyramid <109.5o 13. SbCl5 0 5 sp3d trig. bipyramid 120o & 90o 14. XeF2 3 2 sp3d linear 180o 0 3 (sites) sp2 trig. planar 120o 2 4 sp3d2 square planar 90o 10. I3 11. XeF6 +2 12. SeBr3 15. CO3 -2 16. XeF4 +1 ------------------------------------Rank the bond angles of H2O, H2S, H2Se, and H2Te from largest to smallest. Bond angle of H-O-H (104.5o) > H-S-H (~92o) > H-Se-H (~91o) > H-Te-H (~90o) Explain why you ranked them as you did. As the size of the central atom increased, the lone pair electrons were able to exert a greater repulsion.