FDA Consumer magazine
advertisement

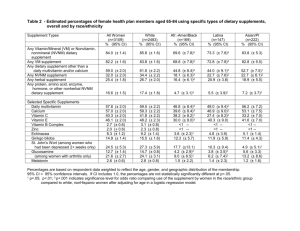
FDA Consumer magazine Online Use Caution Buying Medical Products By Michelle Meadows needed! Cure cancer with herbs! Zap your pain Get prescription drugs fast--no doctor Absolutely safe--pull out your credit card NOW, AND get !away with an amazing device .rock-bottom prices And if they " .It's not hard to find statements like these floating around in cyberspace Rich Cleland, assistant sound too good to be true, it's because they usually are," says .)Federal Trade Commission (FTC director of the Division of Advertising Practices at the health products with the benefits of Many legitimate Web sites bring customers cheaper prices. "But consumers need to be ,convenience, privacy, and, sometimes created a marketplace for unapproved medical products, aware that the Internet has also products marketed with fraudulent health claims," says William illegal prescribing, AND associate commissioner for policy AND planning at the Food AND Drug ,Hubbard .Administration broad reach, relative anonymity, And the unique qualities of the Internet, including its" sites, pose challenges for enforcing AND the ease of creating AND removing Web Many sites are connected to other sites AND " .federal AND state laws," Hubbard says investigations more complex. AND there are have multiple links, which makes regulatory AND enforcement issues cross state, jurisdictional challenges because the ".lines federal, AND international prosecute Government agencies work together to shut down illegal Web sites AND take some criminals, but enforcement resources are limited. "Consumers need to way," Hubbard says. responsibility for recognizing suspicious sites AND turning the other you protect your health AND So how can you spot the red flags? Here's a guide to help your wallet . Dietary Supplements .they? Dietary supplements are products taken as a supplement to the diet What are individual Examples are vitamins, minerals, herbs, botanicals, AND amino acids, the are classified as foods building blocks of proteins needed for all life. Dietary supplements .AND not drugs that dietary supplements will prevent, treat, OR Problem sites: Web sites cannot claim make the product an unapproved AND illegal drug. Also, cure any disease. This would claims that a dietary supplement will have an effect on any Web sites can't make .function of the body when the claims are not substantiated structure OR selling dietary supplements with false OR unsubstantiated claims sometimes Web sites" testimonials AND advertisements touting a quick, miracle cure," says the FTC's use cancer, arthritis, ,Cleland. "And some sites claim a product will cure it all--heart disease ".you name it claims for major diseases AND weight loss. Cleland says he sees a lot of miracle fears about terrorism," he adds. After the anthrax "Criminals also prey on people's falsely claimed that dietary supplements such as colloidal attacks in 2001, some sites .oil could protect against biological AND chemical contamination silver AND oregano promoting some products, companies are telling patients not to undergo Risks: "In chemotherapy, OR other needed treatment," says Cleland. "So we are ,surgery ".about people forgoing legitimate medical treatment concerned worry about ingesting harmful substances. Companies may call Consumers also have to natural," but that doesn't mean it's safe. AND dietary supplements are " a product to supplement diets, not replace them. Too much of some nutrients can cause intended drugs you problems. There is also a danger of dietary supplements interacting with other supplement gingko biloba, may be taking. The prescription medicine warfarin, the herbal taking any of them together can increase aspirin, AND vitamin E all can thin the blood, so .the potential for internal bleeding DSHEA), ( Regulation: Under the Dietary Supplement Health AND Education Act of 1994 diet AND that dietary supplements are products that are intended to supplement the vitamin, a mineral, an herb contain one OR more of the following dietary ingredients: a substance that supplements the diet by OR other botanical, an amino acid, a dietary concentrate, metabolite, constituent, extract, OR increasing the total daily intake, OR a .ingredients combination of these days before marketing 75 Dietary supplement manufacturers must notify the FDA at least This includes providing the agency ".products containing some "new dietary ingredients New dietary ingredients are those that .with safety information about the supplement .before Oct. 15, 1994 were not marketed as dietary supplements dietary ingredients, the safety AND Except for those dietary supplements containing new monitored only after they reach the marketplace. labeling of most dietary supplements is dietary supplements after they are on the market, The FDA evaluates the safety of manufacturing, AND product information on the labeling. The FTC ,overseeing safety advertising of dietary supplements under the FTC Act, which prohibits regulates the .claims in advertising deceptive showing a dietary supplement is Under DSHEA, the FDA generally has responsibility for product's use. For example, in 2004, the unsafe before it can take action to restrict the alkaloids in dietary supplements because the FDA banned the use of ephedrine risk of illness OR injury. Ephedrine alkaloids in dietary substances pose an unreasonable .linked to cardiovascular problems supplements have been false OR misleading, OR if a firm is making drug If the FDA can establish that claims are" supplement, the agency can take action using any of our enforcement claims for a dietary as warning letters, cyber letters, seizure of products, AND criminal tools, such labeling prosecution," says Jennifer Thomas, who leads the dietary supplement AND Nutrition, Office of enforcement team in the FDA's Center for Food Safety AND Applied .Compliance to diseases OR conditions that are Consumers should be wary of claims related Severe Acute Respiratory Syndrome (SARS) prominent in the news. For example, when several dietary supplement products promoted was in the news in 2003, the FDA found preventing SARS. The FDA took action against 10 of on the Internet for treating OR evidence of safety OR effectiveness of the products for use these firms, as there was no .against SARS :Enforcement Examples street drug alternative products called Since 2003, the FDA has taken action against • seizing millions of dollars worth of these ","Black Beauties" AND "Yellow Jackets marketed as dietary supplements, such products are products. Although labeled AND .cannot be sold as dietary supplements actually unapproved drugs AND consumers against purchasing a liquid product In February 2004, the FDA warned • was promoted on the Internet AND sold in stores as a called "Green Hornet." Although it product was actually an illegal drug because it was promoted as dietary supplement, the version of Ecstasy. After taking the product, four teen-agers were rushed to the an herbal blood hospital with seizures, excessive heart rates, severe body rashes, AND high .pressure SeaSilver USA Inc. AND In March 2004, the FDA AND the FTC announced that • decree of permanent injunction Carlsbad, Calif., signed a consent Americaloe Inc. of liquid supplement called SeaSilver AND agreed to stop manufacturing a bogus cure-all .AND other products FDA announced the sentencing of a man who swindled cancer In June 2004, the • advertising AND selling Laetrile, also known as vitamin B-17 OR patients by heavily Although he purported it to be a dietary supplement, Laetrile is actually an .apricot pits .cancer unapproved drug. The highly toxic product hasn't shown any effect on treating September 2004, The FDA issued a warning letter to Cellular Wellness Foundation in • was effective in treating citing claims made on its Web site that the product Cellular Tea .serious diseases such as cancer FDA issued warning letters to 25 firms that promote their products on the In 2004, the • are not Internet with claims that the products are useful for weight loss. The claims .supported by scientific evidence Court for the District of New Jersey found that three In July 2004, the U.S. District • Labs-USA Inc. AND its president Andrew J. Lane as dietary products sold by Lane cosmetic--Benefin, MGN-3, AND SkinAnswer--are in fact supplements AND a federal law because they were being marketed as unapproved new drugs under skin cancer without FDA approval. The court treatments for cancer, HIV, AND company from distributing Benefin, MGN-3, AND permanently enjoined Lane AND the either approved for marketing by the FDA OR distributed SkinAnswer unless they are first investigational new drug application for purposes of conducting a clinical pursuant to an the three trial. The court also ordered the defendants to pay restitution to purchasers of .court's decision products since Sept. 22, 1999. The defendants are appealing the the Internet should consider Tips: Consumers who choose to buy dietary supplements on .provided to substantiate claims who operates the Web site AND what evidence is for making sure that their products are safe Dietary supplement makers are responsible that claims on labels are accurate, truthful, AND before they go on the market AND scientific evidence. By law, supplement manufacturers are substantiated with adequate :types of claims, when appropriate allowed to use these ".in calcium" OR "excellent source of vitamin C Nutrient-content claims such as "high • link between the supplement AND reduced risk of a disease Health claims that show a • when the use of the claim has been approved by the FDA. For ,OR health condition get adequate amounts of the B vitamin folic acid during pregnancy example, women who .decreased risk of having a baby with a neural tube defect have a health claims, which are for dietary supplements only AND came about as a Qualified • Circuit of a 1999 decision by the U.S. Court of Appeals for the District of Columbia result to allow in the case of Pearson v. Shalala. The court's ruling requires the FDA such qualification. appropriately qualified health claims that would be misleading without scientific evidence. An example of These qualified claims are based on the weight of the conclusive research shows that consumption of this type of claim is "supportive but not DHA (docosahexaenoic acid) omega-3 fatty acids EPA (eicosapentaenoic acid) AND ".heart disease may reduce the risk of coronary deficiency disease, such as Claims regarding a benefit related to a classical nutrient • .vitamin C AND scurvy supplement has an effect on the structure OR function of the body, Claims that a dietary • claims are supported by scientific evidence. An example of such a claim is when such .calcium builds strong bones" for a supplement that contains calcium" .Claims that describe general well-being from consumption of the product • FDA recommends that consumers contact their health care providers before using The breast- dietary supplements. This is especially important for people who are pregnant OR over-the-counter feeding, chronically ill, elderly, under 18, OR taking prescription OR .medicines