student_notes_2012_Unit_ 2
advertisement

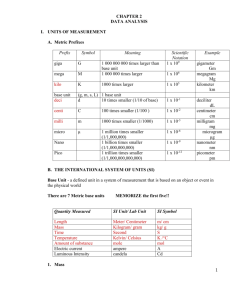
CHAPTER 2 DATA ANALYSIS I. UNITS OF MEASUREMENT A. Metric Prefixes Prefix Symbol Meaning Scientific Notation 1 x 109 giga G mega M 1 000 000 000 times larger than base unit 1 000 000 times larger kilo K 1000 times larger 1 x 103 base unit deci (g, m, s, L) 1 base unit d 10 times smaller (1/10 of base) 1 x 10-1 centi c 100 times smaller (1/100 ) 1 x 10-2 milli m 1000 times smaller (1/1000) 1 x 10-3 1 x 106 Example gigameter Gm megagram Mg kilometer km deciliter dL centimeter cm milligram mg microgram μg nanometer nm picometer pm 1 million times smaller 1 x 10-6 (1/1,000,000) nano n 1 billion times smaller 1 x 10-9 (1/1,000,000,000) pico p 1 trillion times smaller 1 x 10-12 (1/1,000,000,000,000) B. THE INTERNATIONAL SYSTEM OF UNITS (SI) Base Unit - a defined unit in a system of measurement that is based on an object or event in the physical world There are 7 Metric base units MEMORIZE the first five!! micro µ Quantity Measured SI Unit/ Lab Unit SI Symbol Length Mass Time Temperature Amount of substance Electric current Luminous Intensity Meter/ Centimeter Kilogram/ gram Second Kelvin/ Celsius Mole Ampere Candela m/ cm kg/ g s K /°C mol A Cd 1. Mass a) the amount of matter in a substance [recall that weight is a measure of the force of gravity between two objects] b) kilogram (kg) is official base unit [too large for chemistry so we use gram (g)] c) measured on a balance 1 2. Length a) distance covered by a straight line connecting two points b) meter (m) is base unit cm is the unit we use in lab c) measured with a ruler or similar device 3. Time a) the interval between two occurrences b) measured in seconds (s) c) measured with clock or watch 4. Temperature a) the measure of the average kinetic energy of the particles of a sample b) lab temperature scale (Celsius, °C): 0° C = freezing point of water at 1 atmosphere of pressure 100°C = boiling point of water at 1 atmosphere of pressure room temperature = about 25°C body temperature = about 37°C c) metric absolute temperature scale (Kelvin, K) 0 K = absence of all molecular motion (absolute zero) 273 K = freezing point of water 373 K = boiling point of water 298 K = room temperature d) Related to Celsius by: °C + 273 = K II. DERIVED UNIT - a unit that is defined as a combination of base units A. Volume = V • measure of the amount of space matter occupies • measured in cm3 or mL 1 cm3 = 1 mL (MEMORIZE THIS) • if liquid, measure in a graduated cylinder: read bottom of meniscus • if regular solid, measure with ruler: length x width x height = V • if irregular solid, measure by water displacement: fill graduated cylinder to specific volume (record) add irregular shaped object record the new volume subtract: final vol. - initial vol. = object vol. B. Density (to be discussed at end of chapter) III. Taking Measurements and Accuracy and Precision A. Accuracy - how close a measurement is to the true value B. Precision - how close a series of measurements are to each other C. Percent Error Percent Error = |experimental value - "book" value| x 100% 2 "book" value always positive number 3 3 Ex. A students finds the density of water to be 0.92 g/cm . The actual value is 1.00 g/cm . What is the percent error of my value? D. Taking Measurements (from Laying the Foundation) The accuracy of a measurement depends on two factors: the skill of the individual taking the measurement and the capacity of the measuring instrument. When making measurements, you should always read to the smallest mark on the instrument and then estimate another digit beyond that. For example, if you are reading the length of the steel pellet pictured above using only the ruler shown to the left of the pellet, you can confidently say that the measurement is between 1and 2 cm. However, you MUST include one additional digit estimating the distance between the 1 and 2 cm marks. The correct measurement for this ruler should be reported as 1.5 cm. It would be incorrect to report this measurement as 1 cm or even 1.50 cm given the scale of this ruler. What if you are using the ruler shown on the right of the pellet? What is the correct measurement of the steel pellet using this ruler? 1.4 cm? 1.5 cm? 1.40 cm? 1.45 cm? The correct answer would be 1.45 cm. Since the smallest markings on this ruler are in the tenths place we must carry our measurement out to the hundredths place. If the measure value falls exactly on a scale marking, the estimated digit should be zero. The temperature on this thermometer should read 30.0°C. A value of 30°C would imply this measurement had been taken on a thermometer with markings that were 10°C apart, not 1°C apart. When using the instruments with digital readouts you should record all the digits shown. The instrument has done the estimating for you. When measuring liquids in narrow glass graduated cylinders, most liquids form a slight dip in the meniscus. Plastic graduated cylinders do not usually have a meniscus. In this case you should read the cylinder from the top of the liquid surface. Practice reading the volume contained in the 3 cylinders below. Record your values in the space provided. 3 IV. CRITICAL MATH SKILLS A. Math Review --- Getting to know your calculator 1. Parentheses try this: 4(3 + 5) or: 4.184 x 10.0 (100.0 - 92.1) 2. Scientific Notation a) use for very large (>1000) or very small numbers (<.001) b) LARGE NUMBERS have POSITIVE exponents small numbers have negative exponents c) Standard scientific notation: only one number in front of the decimal point d) Self-Test Put these numbers in scientific notation. a) 40,230,000 b) 0.0099 c) 7.3 x 10-3 d) 3.18 x 10-4 Now let's add c and d on your calculator. On your calculator, punch 7.3 2nd comma button( this is the EE button) then punch (+/-) button (to the left of Enter) and then 3 plus 3.18 2nd comma button then punch (+/-) and then 4. Some of you may be used to using the button. This does not work all the time. Get use to using the 2nd comma button. B. Significant Figures • only represents numbers actually measured and one estimate position • depends on the instrument's precision RULES 1. All non-zero digits are significant Ex1: 456 ____ Ex2: 932.76 _____ 2. Leading zeros are NEVER significant – leading zeros are all zeros in front of the first whole number. Ex3: 0.0000234 _____ Ex4. 0.002 ______ 4 3. Middle zeros are ALWAYS significant Ex5: 1002 _____ Ex6: 9.0043 _______ 4. Trailing zeros are significant ONLY IF THERE IS A DECIMAL POINT IN THE NUMBER Trailing zeros are all zeros behind the last whole number. Ex7: 223.0 _______ Ex8: 200 ________ Ex9: 9.87000 ________ Rounding Off 1. Calculations with measurements must maintain proper degree of certainty. 2. Rules: a. In multiplication and division, the answer may not contain any more SIGNIFICANT DIGITS than the number in the calculation with the fewest significant digits. ex: 1.5 grams = 0.375 g/mL Round this to __________ 4 b. In addition and subtraction, the answer may not contain any more DECIMAL PLACES than the number in the calculation with the fewest decimal places. ex. 98 + 213.67 311.67 Round this to: ________ V. DENSITY A. Density = mass per unit volume = D = m/V B. Units of density D = m = g or g 3 V mL cm C. Density problems ex. #1 - mass = 4.98 g volume = 2.36 mL density = ? ex. #2 - mass = 3.2 g length = 2 cm width = 2 cm height = 2 cm volume = ?density = ? ex. #3 - mass = 7.8 g initial water level = 10.0 mL final water level = 17.4 mL volume = ? density = ? ex #4 - density = 1.02 g/mL volume = 3.45 mL ex. #5 - density = 2.1 g/mL mass = ? g mass = 3.5 g volume = ? mL 5 VI. REPRESENTING DATA GRAPHING A graph is a visual display of data. Types of Graphs Circle Graph, also called a pie chart. Bar Graph Line Graph Points on a line graph represent the intersection of data for two variables Independent variable, variable which the scientist deliberately changes during and experiment, is plotted on the x-axis. Dependent variable is plotted on the y-axis 6 Best fit line is a line that must be drawn so that about as many points fall above the line as fall below it. If the best fit line is straight the independent and dependent variables are directly related. This can be described by the slope of the line. If the line rises to the right, the slope is positive. Positive slope indicates that the dependent variable increases as the independent variable increases. If the line sinks to the right, the slope is negative and indicates that the dependent variable decreases as the independent variable increases. Slope can be calculated using the following equation: Slope = y2 – y1 x2 – x1 Line equation: y=mx + b where b=y-intercept Interpreting graphs Interpolation – reading data from a graph that falls between measured points Extrapolation – extending the line beyond the plotted points and estimate values for the variables 7