teacher notes Chap. 2.1 thru 2.4
advertisement

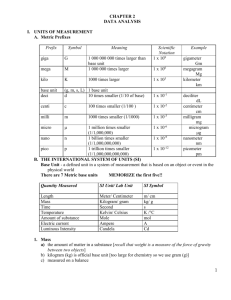
CHAPTER 2 DATA ANALYSIS I. UNITS OF MEASUREMENT A. Metric Prefixes Prefix Symbol Meaning Scientific Notation 1 x 109 giga G mega M 1 000 000 000 times larger than base unit 1 000 000 times larger kilo K 1000 times larger 1 x 103 base unit deci (g, m, s, L) 1 base unit d 10 times smaller (1/10 of base) 1 x 10-1 centi C 100 times smaller (1/100 ) 1 x 10-2 milli m 1000 times smaller (1/1000) 1 x 10-3 micro µ 1 million times smaller (1/1,000,000) 1 billion times smaller (1/1,000,000,000) 1 trillion times smaller (1/1,000,000,000,000) 1 x 10-6 Nano Pico 1 x 106 1 x 10-9 1 x 10-12 Example gigameter Gm megagram Mg kilometer km deciliter dL centimeter cm milligram mg microgram μg nanometer nm picometer pm B. THE INTERNATIONAL SYSTEM OF UNITS (SI) Base Unit - a defined unit in a system of measurement that is based on an object or event in the physical world There are 7 Metric base units MEMORIZE the first five!! Quantity Measured SI Unit/ Lab Unit SI Symbol Length Mass Time Temperature Amount of substance Electric current Luminous Intensity Meter/ Centimeter Kilogram/ gram Second Kelvin/ Celsius mole ampere candela m/ cm kg/ g S K /°C mol A Cd 1. Mass 1 a) the amount of matter in a substance [recall that weight is a measure of the force of gravity between two objects] b) kilogram (kg) is official base unit [too large for chemistry so we use gram (g)] c) measured on a balance 2. Length a) distance covered by a straight line connecting two points b) meter (m) is base unit cm is the unit we use in lab c) measured with a ruler or similar device 3. Time a) the interval between two occurrences b) measured in seconds (s) c) measured with clock or watch 4. Temperature a) the measure of the average kinetic energy of the particles of sample b) metric temperature scale (Celsius, °C): 0° C = freezing point of water at 1 atmosphere of pressure 100°C = boiling point of water at 1 atmosphere of pressure room temperature = about 25°C body temperature = about 37°C c) absolute temperature scale (Kelvin, K) 0 K = absence of all molecular motion (absolute zero) 273 K = freezing point of water 373 K = boiling point of water 298 K = room temperature d) Related to Celsius by: °C + 273 = K II. a DENSITY DERIVED UNIT - a unit that is defined as a combination of base units Density = mass per unit volume = D = m/V A. Mass = m • measured in grams • measured on a balance • may be calculated "by difference" mass the empty container first add desired material mass the full container subtract to find mass of material B. Volume = V • measure of the amount of space matter occupies • measured in cm3 or mL 2 • • • C. D. 1 cm3 = 1 mL (MEMORIZE THIS) if liquid, measure in a graduated cylinder: read bottom of meniscus if regular solid, measure with ruler: length x width x height = V if irregular solid, measure by water displacement: fill graduated cylinder to specific volume (record) add irregular shaped object record the new volume subtract: final vol. - initial vol. = object vol. Units of density D = m = g or V mL g cm3 Density problems ex. #1 - density of a liquid mass = 4.98 g volume = 2.36 mL density = ? ex. #2 - density of a regular solid cube mass = 3.2 g length = 2 cm width = 2 cm height = 2 cm volume = ? density = ? ex. #3 - density of an irregular solid mass = 7.8 g initial water level = 10.0 mL final water level = 17.4 mL volume = ? density = ? E. Using the density formula • rearrange the basic formula to find mass or volume • plug data into the new formula; solve ex #1 - finding mass D=m/V so D•V = m density = 1.02 g/mL volume = 3.45 mL mass = ? g ex. #2 - finding volume 3 D=m/V, so V = m/D density = 2.1 g/mL mass = 3.5 g volume = ? mL III. CRITICAL MATH SKILLS INTRODUCTION: It is possible to study chemistry and gain some appreciation and understanding of it without mathematics. However, the depth of your comprehension of chemistry (and almost every other subject) is directly related to your math skills that you should master before studying chemistry. It is critical that you seek tutoring at any time you do not understand a topic in this unit. A. Math Review --- Getting to know your calculator 1. Parentheses try this: 4(3 + 5) or: 4.184 x 10.0 (100.0 - 92.1) 2. Scientific Notation a) use for very large (>1000) or very small numbers (<.001) (more than 3 digits) b) LARGE NUMBERS have POSITIVE exponents small numbers have negative exponents c) Standard scientific notation: only one number in front of the decimal point d) Self-Test Put these numbers in scientific notation. a) 40,230,000 b) 0.0099 Write these numbers in ordinary form: a) 7.3 x 10-3 b) 3.18 x 104 Now let's add a and b on your calculator. On your calculator, punch 7.3 2nd comma button( this is the EE button) then punch (-) button (to the left of Enter) and then 3 plus 3.18 2nd comma button 4. Some of you may be used to using the button. This does not work all the time. Get use to using the 2nd comma button. 4 IV. V. Accuracy and Precision Accuracy • how close a measurement is to the true value Precision • how close a series of measurements are to each other Percent Error • use when determining an experimental value for quantity that is already known. • used to compare your value to the "book" or known value Percent Error = |experimental value - "book" value| x 100% "book" value • always positive number ex. I find the density of water to be 0.92 g/cm3. The actual value is 1.00 g/cm3. What is the percent error of my value? VI. Significant Figures • only represents numbers actually measured and one estimate position • depends on the instrument's precision RULES 1. All non-zero digits are significant EX: 456 932.76 2. Leading zeros are NEVER significant EX: 0.0000234 0.002 3. Middle zeros are ALWAYS significant EX: 1002 9.0043 4. Trailing zeros are significant ONLY IF THERE IS A DECIMAL POINT IN THE NUMBER EX: 223.0 200 9.87000 5. Counting numbers and defined constants have an infinite number of significant figures. EX: 6 molecules 60 s =1 min 5 Rounding Off 1. Calculations with measurements must maintain proper degree of certainty. 2. Rules: a. In multiplication and division, the answer may not contain any more SIGNIFICANT DIGITS than the number in the calculation with the fewest significant digits. ex: 1.5 grams = 0.375 g/mL 4 mL ROUND THIS TO: 0.4 g/mL b. In addition and subtraction, the answer may not contain any more DECIMAL PLACES than the number in the calculation with the fewest decimal places. ex. VII. 98 + 213.67 311.67 ROUND THIS TO: 312 REPRESENTING DATA GRAPHING A graph is a visual display of data. Types of Graphs Circle Graph, also called a pie chart. 6 Bar Graph Line Graph Points on a line graph represent the intersection of data for two variables Independent variable, variable which the scientist deliberately changes during and experiment, is plotted on the x-axis. Dependent variable is plotted on the y-axis Best fit line is a line that must be drawn so that about as many points fall above the line as fall below it. If the best fit line is straight the independent and dependent variables are directly related. This can be described by the slope of the line. If the line rises to the right, the slope is positive. Positive slope indicates that the dependent variable increases as the independent variable increases. If the line sinks to the right, the slope is negative and indicates that the dependent variable decreases as the independent variable increases. Slope can be calculated using the following equation: Slope = y2 – y1 x2 – x1 Interpreting graphs Interpolation – reading data from a graph that falls between measured points Extrapolation – extending the line beyond the plotted points and estimate values for the variables 7 8