Practice Exam 2
advertisement
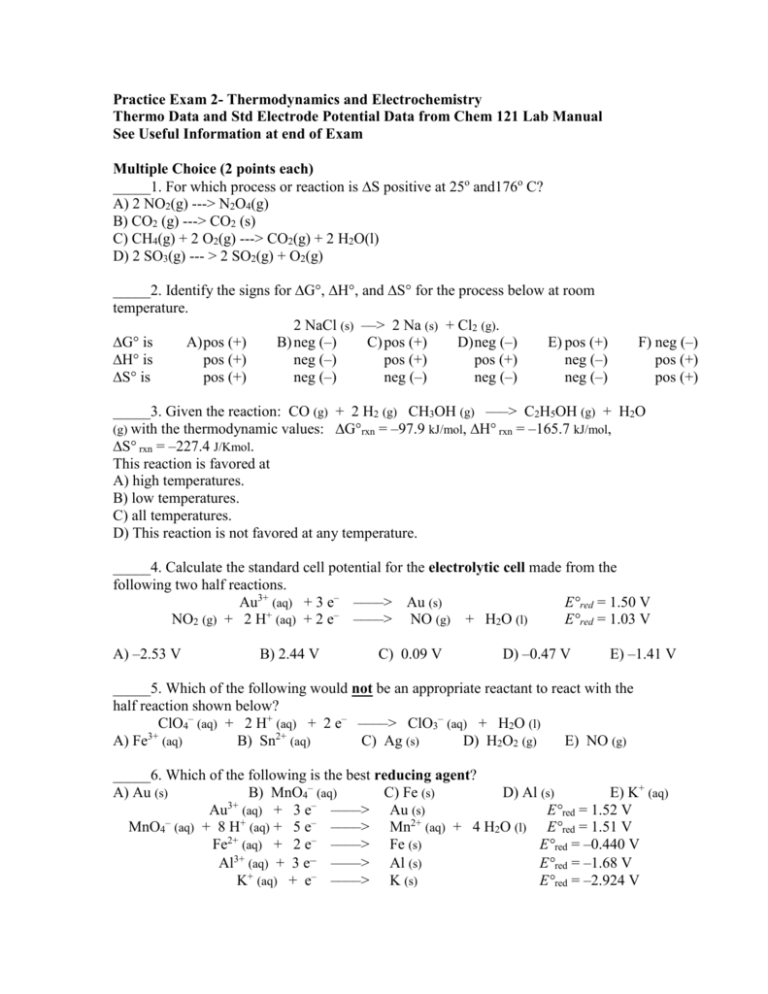
Practice Exam 2- Thermodynamics and Electrochemistry Thermo Data and Std Electrode Potential Data from Chem 121 Lab Manual See Useful Information at end of Exam Multiple Choice (2 points each) _____1. For which process or reaction is S positive at 25o and176o C? A) 2 NO2(g) ---> N2O4(g) B) CO2 (g) ---> CO2 (s) C) CH4(g) + 2 O2(g) ---> CO2(g) + 2 H2O(l) D) 2 SO3(g) --- > 2 SO2(g) + O2(g) _____2. Identify the signs for ∆G°, ∆H°, and ∆S° for the process below at room temperature. 2 NaCl (s) ––> 2 Na (s) + Cl2 (g). ∆G° is A) pos (+) B) neg (–) C) pos (+) D) neg (–) E) pos (+) ∆H° is pos (+) neg (–) pos (+) pos (+) neg (–) ∆S° is pos (+) neg (–) neg (–) neg (–) neg (–) F) neg (–) pos (+) pos (+) _____3. Given the reaction: CO (g) + 2 H2 (g) CH3OH (g) –––> C2H5OH (g) + H2O (g) with the thermodynamic values: ∆G°rxn = –97.9 kJ/mol, ∆H° rxn = –165.7 kJ/mol, ∆S° rxn = –227.4 J/Kmol. This reaction is favored at A) high temperatures. B) low temperatures. C) all temperatures. D) This reaction is not favored at any temperature. _____4. Calculate the standard cell potential for the electrolytic cell made from the following two half reactions. Au3+ (aq) + 3 e– ––––> Au (s) E°red = 1.50 V + – NO2 (g) + 2 H (aq) + 2 e ––––> NO (g) + H2O (l) E°red = 1.03 V A) –2.53 V B) 2.44 V C) 0.09 V D) –0.47 V E) –1.41 V _____5. Which of the following would not be an appropriate reactant to react with the half reaction shown below? ClO4– (aq) + 2 H+ (aq) + 2 e– ––––> ClO3– (aq) + H2O (l) A) Fe3+ (aq) B) Sn2+ (aq) C) Ag (s) D) H2O2 (g) E) NO (g) _____6. Which of the following is the best reducing agent? A) Au (s) B) MnO4– (aq) C) Fe (s) D) Al (s) E) K+ (aq) Au3+ (aq) + 3 e– ––––> Au (s) E°red = 1.52 V – MnO4 (aq) + 8 H+ (aq) + 5 e– ––––> Mn2+ (aq) + 4 H2O (l) E°red = 1.51 V Fe2+ (aq) + 2 e– ––––> Fe (s) E°red = –0.440 V 3+ – Al (aq) + 3 e ––––> Al (s) E°red = –1.68 V K+ (aq) + e– ––––> K (s) E°red = –2.924 V _____7. Which of the following changes below, if any, could create spontaneous conditions? 2 H+ (aq) + 2 Br– (aq) + 2 NO2 (g) –––> Br2 (l) + 2 HNO2 (aq) ∆G° = + 4.4 kJ – A) add OH (aq) B) remove Br– (aq) C) increase the pressure of NO2 (g) D) none of the above, the ∆G° is nonspontaneous E) all of the above _____8. A voltaic cell prepared using aluminum and nickel has the following cell notation. Al(s) | Al3+(aq) || Ni2+(aq) | Ni(s) Which of the following represents the correctly balanced spontaneous reaction equation for the cell? A) Ni2+(aq) + Al(s) Al3+(aq) + Ni(s) B) 3Ni2+(aq) + 2Al(s) 2Al3+(aq) + 3Ni(s) C) Ni(s) + Al3+(aq) Ni2+(aq) + Al(s) D) 3Ni(s) + 2Al3+(aq) 3Ni2+(aq) + 2Al(s) _____9. Consider a voltaic cell based on these half-cells. Ag+(aq) + e¯ ---> Ag(s) E° = +0.80 V Cd2+(aq) + 2e¯ ---> Cd(s) E° = -0.40 V Identify the anode and give the voltage of this cell under standard conditions. A) Ag; Ecell = 0.40 V B) Ag; Ecell = 2.00 V C) Ag; Ecell = 1.20 V D) Cd; Ecell = 1.20 V E) Cd; Ecell = 2.00 V F) Cd; Ecell = 0.40 V _____10. Zn(s) + Cl2(g, 1 atm) Zn2+(aq, 1 M) + 2Cl¯(aq, 1 M) An electrochemical cell based on this reaction has a cell voltage, E°, of 2.12 V. Which change could make the cell voltage greater than 2.12 V? A) add more Zn(s) B) add more Cl¯(aq) ions C) decrease the concentration of Zn2+(aq) ions D) decrease the partial pressure of Cl2 E) more than one of the above 11. (4 pts) Write the reactions that will occur when molten CuCl2 is electrolyzed. A) cathode reaction B) anode reaction 12. (9 pts) Given a galvanic cell where one of the operating half reaction is: Pb (s) + 3 OH– (aq) ––––> HPbO2– (aq) + H2O (l) + 2 e– A) State a reasonable material to be used for the electrode. ________________ B) Draw the half cell in detail and show the electrochemistry that is taking place including the electron flow. C) Identify the change in mass, if any, at the electrode. Explain any changes in mass or why there is no change in electrode mass. 13. (10 pts) What is the cell potential for the reaction below buffered at a pH of 10.00 and having an Al(OH)4– (aq) concentration of 3.5 M? Each solid has a mass of 20 g. The cell is operated at 25oC. 2 Al (s) + 2 OH– (aq) + 3 Zn(OH)2 (s) ––––> 2 Al(OH)4– (aq) + 3 Zn (s) 14. (8 pts) Calculate Ksp for Hg2Cl2 at 298 K given Hg2Cl2(s) + 2e- 2Hg(l) + 2Cl-(aq) Eo = 0.34 V 2+ Hg2 (aq) + 2e 2Hg(l) Eo = 0.80 V 15. (8 pts) Calculate the number of minutes an electroplating apparatus would have to operate at a current of 4.00 A to plate out the platinum from 0.50 L of a 0.100 M PtCl4 solution. 16. (12 pts) A voltaic cell is constructed in the following manner: Ag (s) | Ag+ (sat’d AgI) || Ag+ (sat’d AgCl, x M Cl–) | Ag (s) What does the concentration of Cl– in the cathode need to be if you want the cell to have a measured cell potential of 0.0860V? 17. (13 pts) Consider this reaction for the combustion of ethanol. The H° = - 1234.7 kJ and the S° = 251.5 J/K at 25°C C2H5OH(l) + 3O2(g) ---> 2CO2(g) + 3H2O(g) A) Calculate the value of the equilibrium constant, Keq, for this combustion reaction at 25oC. B) If the water formed as a product in this reaction appeared as a liquid rather than as a gas, how would H for the reaction be expected to change? Explain your answer. 18. (16 pts) The following question relates to the vaporization of methanol, CH3OH, CH3OH(l) CH3OH(g) Use the following data in answering this question Substance Hfo (kJ/mol) Gfo (kJ/mol) So (J/mol.K) CH3OH(g) -201 -163 240. CH3OH(l) -239 -166 127 o o A) Calculate G at 60. C. B) What is the vapor pressure of methanol at 60 oC? C) What is the normal boiling point of methanol? Useful Information Constants R = 8.3145 J/mol.K = 0.08206 L.atm / mol.K 1 J = 1 kg.m2/s2 F = 96500 C/mol e1 amu = 1.66054 x 10-27 kg = 931.5 MeV mass of an electron = 5.4857x10-4 amu mass of a proton = 1.007277 amu mass of a neutron = 1.008665 amu charge on an electron = 1.60 x 10-19 C c = 2.998x108 m/s h = 6.63 x 10-34 J.s N = 6.022 x 1023 Equations h c E = q + W W = -PV Suniv = Ssurroundings + Ssystem G = H – TS G = Go + RTlnQ Go = - RTlnK ln K 2 ln K1 RH at 25 oC 1 T2 T11 E = Eo – 0.0592 logQ n I = q/t Integrated Rate Laws Zero Order [A] = [Ao] – kt First Order ln[A] = -kt + ln[Ao] Second Order 1 = kt + 1 [A] [A]o k = Ae-Ea/RT 1 1 ln k 2 E a R T 2 T 1 k1 E = mc2 G = -nFE Eo = 0.0592 logK n







