Practice Exam 4 – Kinetics and Nuclear Chemistry
advertisement
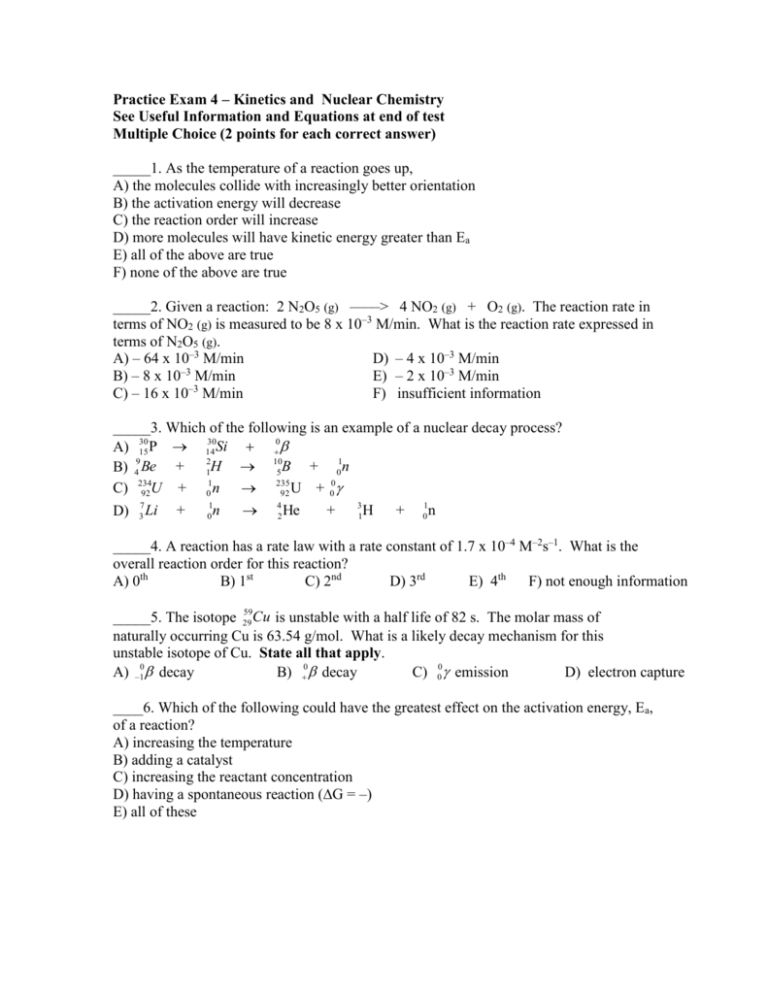
Practice Exam 4 – Kinetics and Nuclear Chemistry See Useful Information and Equations at end of test Multiple Choice (2 points for each correct answer) _____1. As the temperature of a reaction goes up, A) the molecules collide with increasingly better orientation B) the activation energy will decrease C) the reaction order will increase D) more molecules will have kinetic energy greater than Ea E) all of the above are true F) none of the above are true _____2. Given a reaction: 2 N2O5 (g) ––––> 4 NO2 (g) + O2 (g). The reaction rate in terms of NO2 (g) is measured to be 8 x 10–3 M/min. What is the reaction rate expressed in terms of N2O5 (g). A) – 64 x 10–3 M/min D) – 4 x 10–3 M/min –3 B) – 8 x 10 M/min E) – 2 x 10–3 M/min C) – 16 x 10–3 M/min F) insufficient information _____3. Which of the following is an example of a nuclear decay process? 30 14 Si 0 A) 30 15 P B) 49 Be + 12H 105 B + 01n + 01n 235 + 00 C) 234 92 U 92 U D) 7 3 Li + 1 0 n 4 2 He + 3 1 H + 1 0 n _____4. A reaction has a rate law with a rate constant of 1.7 x 10–4 M–2s–1. What is the overall reaction order for this reaction? A) 0th B) 1st C) 2nd D) 3rd E) 4th F) not enough information 59 Cu is unstable with a half life of 82 s. The molar mass of _____5. The isotope 29 naturally occurring Cu is 63.54 g/mol. What is a likely decay mechanism for this unstable isotope of Cu. State all that apply. A) –10 decay B) 0 decay C) 00 emission D) electron capture ____6. Which of the following could have the greatest effect on the activation energy, Ea, of a reaction? A) increasing the temperature B) adding a catalyst C) increasing the reactant concentration D) having a spontaneous reaction (∆G = –) E) all of these _____7. The reaction A B is first-order overall and first-order with respect to the reactant A. The result of doubling the initial concentration of A will be to A) shorten the half-life of the reaction B) lengthen the half-life of the reaction B) increase the rate constant of the reaction C) decrease the rate constant of the reaction E) double the initial rate Please refer to the following mechanism for problems 8-10. OCl– + H2O <–––––> HOCl + OH– I– + HOCl <–––––> HOI + Cl– HOI + OH– <–––––> H2O + OI– _____8. Which of the species shown in the mechanism above is an intermediate? List all that apply. A) OCl– B) H2O C) HOCl D) OH– E) I– – – F) HOI G) Cl H) OI I) none of these _____9. Which of the species shown in the mechanism above is a catalyst? List all that apply. A) OCl– B) H2O C) HOCl D) OH– E) I– F) HOI G) Cl– H) OI– I) none of these _____10. What is the molecularity of the second step in the mechanism? A) unimolecular B) bimolecular C) termolecular D) can not determine 11. (6 pts) For a system that is 0th order, fill in the times that would correspond with the indicated concentrations. Keep it simple and clean! No extensive calculations needed Time passed: 0 100 min __________ __________ __________ [A]: 1.0 M 0.50 M 0.25 M 0.125 M 0.0625 M 12. (7 pts) Balance the following nuclear processes. 246 96 Cm i. ii. iii. 7 3 Li 32 17 + Cl _____________ + 1 0 n 16 4 2 He _________ 254 102 No 5 01n + __________ + + ________ 1 0 n 13. (8 pts) Draw a reaction coordinate diagram for a reaction with the following attributes (characteristics): ∆H°rxn is endothermic, has a two step mechanism, and whose first step is much faster than the second step. Clearly identify the Ea for each step of the reaction in the forward direction. Indicate the transition state(s) and the location(s) of the intermediates. Label the axes. | | | | | | | | | | | | | | |_________________________________________________________________ 14. (4 pts) Sucrose, C12H22O11, decomposes to fructose and glucose in acid solution. When ln [sucrose] is plotted vs. time, a straight line with slope of -0.208 hr-1 results. What is the rate law for the reaction? 15. (8 pts). Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. H+ + H2O2 H2O+–OH (rapid equilibrium) + H2O –OH + Br HOBr + H2O (slow) HOBr + H+ + Br- Br2 + H2O (fast) A) What is the overall reaction equation for this process? B) Determine the rate law for this reaction? 16. (8 pts) For the reaction OCl- + I- OI- + Clthe following data were collected at constant temperature. Initial [OCl-] Initial [I-] Initial [OH-] Initial Rate (mol/L) (mol/L) (mol/L) (mol/(L·min)) 1 0.0040 0.0020 1.00 4.9 10-4 2 0.0020 0.0040 1.00 5.0 x 10-4 3 0.0020 0.0020 1.00 2.5 x 10-4 4 0.0020 0.0020 0.50 5.0 x 10-4 A) Determine the rate law for this reaction. Use appropriate calculations. Trial B) Determine the value and units for the specific rate constant. 17. (8 pts) Butadiene, C4H6 (used to make synthetic rubber and latex paints) dimerizes to C8H12 with a rate law of rate = 0.014 L/(mol·s) [C4H6]2. What will be the concentration of C4H6 after 3.0 hours if the initial concentration is 0.025 M? 18. (10 pts) One method of dating rocks is based on their 87Sr/87Rb ratio. The 87Rb is a beta emitter with a half-life of 5 x 1011years. A certain rock is found to have a ratio of 87 Sr/87Rb of 0.004:1.00. What is the age of the rock? 19. (4 pts) The gas-phase conversion of 1,3-butadiene to 1,5-cyclooctadiene, 2C4H6 C8H12 was studied, providing data shown on the plot alongside, of 1/[butadiene] versus time. Determine the specific rate constant for this reaction. 20. (5 pts) The radioactive isotope tritium decays with a first-order rate constant k of 0.056 year-1. What fraction of the tritium initially in a sample is still present 30. years later? 21. (6 pts) Calculate the binding energy in Joules for 13 H which has an atomic mass of 3.01605 amu. Useful Equations and Information speed of light = 2.9979 x 108 m/s Mass of proton = 1.00728 amu Mass of neutron = 1.00866 amu Mass of electron = 5.48580 x 10-4 amu 1 amu = 1.66 x 10-27kg E = mc2 Rate = - [A] at time t2 – [A] at time t1 t2 – t1 [A] at time t2 – [A] at time t1 t2 – t1 Zero Order Reaction: [At] = [Ao] - kt First Order Reaction: [ ] ln A0 kt [ ] At Second Order Reaction: _1_ = kt + _1__ [At] [A0] Arrhenius Equation 1 1 ln k 2 E a R T 2 T 1 k1 = -[A] t