The Elements
advertisement

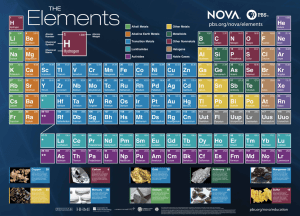
The Elements Name _______________________ Date ________ Bell _____ The ancient Greeks believed that all things were made from combinations of four things: _________________,________________, _____________________, and __________________. We now know that things are made from more basic building blocks, the ___________________. At the time this tape was made, there were ________ elements. The small number is the ____________ number, the number of _______________ in an element measured in ___________ ___________ ____________ or amu. The larger number with a decimal is the atomic __________ (Protons plus neutrons). Hydrogen is the lightest element and has _______ proton. Uranium, the heaviest natural element has _____ protons. In the sun, under tremendous heat and pressure, atoms of hydrogen fuse together to make ________________. This process is called nuclear ____________________. Light is a result of this process. Under even greater pressures on larger stars, hydrogen can fuse to form _______________. On even larger stars, all the elements could be formed. In special nuclear collisions created by man, ________ elements not found in nature have been formed. States on Periodic Table Numbers of elements in that state on the Periodic Table Liquid Gas solids Horizontal rows on the periodic table are called ___________________Vertical columns are called ________________. Member of a _____________ have similar properties. Parts of the atom Clouds of _______________ with a ___________ charge In the nucleus, Protons with a __________ charge, and ______________ with no charge. Air is a mixture of ______________(78%), _______________(21%), and other gases (_____%) These are not chemically united. A compound is chemically bonded elements. An example of this is water, formula __________. The two most common elements are _________________ and __________________. Flames can indicate different elements. Element Color of flame test Carbon Strontium Barium Potassium Copper Sodium I METALS Three quarters of the elements are ____________________. The metals in the middle are called ____________________ metals. We are familiar with the sight of many transition metals like ________________ (Cu), _____________(Ag), __________________ (Au), and ________________ (Fe). The first two groups of metals are the ________________ metals, and the Alkali ____________ metals. These are so reactive, they are never found uncombined in nature. Sodium is very ______________ and begins to tarnish immediately when cut. Sodium chloride is found in table _____________________. Sodium bicarbonate is found in ________________________ and _______________________. Magnesium is an alkaline ________________ metal. It burns _______________ and is found in fireworks. Four special features of metals Metallic property Description of property Reflects light, Can be pounded into sheets Can be stretched into wire Can convey heat and electricity The highest melting point is ____________________, which is used to glow inside light bulbs. The lowest melting point is ______________________, which is used in some thermometers ___________________ is used in high temperature thermometers. Today, Iron is mixed with __________ to make _________________. Iron combines with ___________________ to rust. To prevent this, iron and steel is covered for protection. Bronze is another alloy of copper and _______________. Three magnetic metals are _______________, _______________, and ____________________. Light sensitive compounds are used in photography. II METALLOIDS Silicon can conduct electricity when it is ______ but not when it is cold. Thus it is called a _______-conductor. The “brains” inside of computers are _________________ and _________________. III NONMETALS Important elements for life are often non-metals. We need _______________ to breathe. Our bodies are made of long chains of ____________________ compounds. 99% or our foods are made of ____________, _____________________, and _________________________. One form of carbon is ___________________, used in pencils. Another form of carbon is the _____________________, used in high quality jewelry and abrasives. DNA also contains __________________(N) and _____________________(P) as well as carbon to form the basis of life. ______________________(P) and __________________(S) are combined to make strike anywhere matches. The HALOGENS These are so reactive, they are never found alone in nature. ________________(F) is used in toothpaste. Bleach contains _____________(Cl) to react to make clothes white. Bromine is the only element besides Mercury to be a ___________ at room temperature. Bromine has an odor and the name is Greek for ______________. Iodine does special change of state, going from solid to gas without forming a liquid. This is called ___________________________. The NOBLE GASES ___________________(He) is lighter than air and is used in balloons and blimps. Ordinarily, noble gases are invisible, but when excited by an electrical charge, they can be used in advertising signs. Noble Gas Symbol Color when electrically excited Ne Ar Kr Xe He










![AGE 301 [Farm Mechanics]](http://s3.studylib.net/store/data/009717336_1-d652eb003a5d66f8a922a9f172ff4576-300x300.png)
