Lesson 9
advertisement

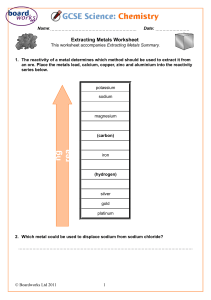
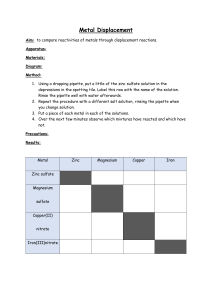
Lesson 9 Uses and sources of metals ILOs to be able to relate the reactivity of metals with their extraction method and use Activities 1) Discuss the uses of metals in the world today and why they are suited to that use. Perhaps include: steel for construction; stainless steel for cutlery; aluminium for aeroplanes; titanium for aerospace and golf clubs; sodium as a coolant in nuclear power stations; gold for jewellery and contact in microchips. Ask some of the following questions: Why is sodium not used for cutlery? Why is a light metal like magnesium not used for car bodies? Why has so much gold jewellery survived from ancient civilisations? Why was bronze used before iron? Aluminium is much more abundant than iron so why was it not used before the beginning of the 20th century? Which metals are found naturally? Why is magnesium not found naturally? How can recycling be used with metals? 2) Demonstrate the thermit reaction to the class. Hazards : Use safety screen as the reaction is violently exothermic. 3) Pupils present their findings from activity 1 by producing an information leaflet linking metals reactivity to their uses, including when they were first used. Page 233 from Spectrum 9 has good information to help with this. The assessment activity can be done at the end of this lesson. Resources Activity 2 3 Assessment activity Resources: Lesson 9 Thermit reaction Spectrum 9 CD-ROM Cards to make word equations