Chemical Formulae
advertisement

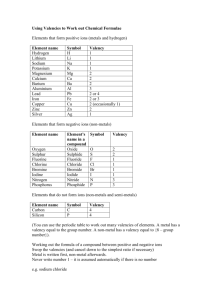
Chemical Formulae Throughout Standard Grade Chemistry, you will be looking at a variety of different topics, ranging from plastic to cow poo! But at the heart of it, Chemistry is all about different atoms joining together. Atoms wish to be like the Nobel Gases (Group 8 or 0), because they have a Full Outer Energy level. e.g. Neon 2, 8 Other atoms can achieve a full outer energy level by losing, gaining or sharing electrons Group 1 e.g. Na 2,8,1 Gives away 1 electron to another atom, so forms ONE BOND Group 2 e.g. Mg 2,8,2 Gives away 2 electrons to other atoms, so forms TWO BONDS Group 3 e.g. Al 2,8,3 Gives away 3 electrons to other atoms, so forms THREE BONDS Group 4 e.g. C 2,4 Has a half-filled energy level, so they don’t gain or lose. Instead they share electrons. They need a share of 4 electrons, so forms FOUR BONDS. Group 5 e.g. N 2,5 Need a share of 3 electrons or needs to gain 3, so forms THREE BONDS Group 6 e.g. O 2,6 Need a share of 2 electrons or needs to gain 2, so forms TWO BONDS Group 7 e.g. F 2,7 Needs a share of 1 electron or needs to gain 1, so forms ONE BOND Group 8 e.g. Ar 2,8,8 Do not need to lose, gain or share electrons, so form NO BONDS!!! The no. of bonds that an atom forms is called its Valency. This is because the bonding of atoms involves its outer electrons, which are formally known as their valence electrons Chemical Formula The no. of each atom in a molecule can be expressed by its chemical formula e.g. Water contains 2 atoms of hydrogen and one atom of oxygen, so its formula is H2O Using the valencies of elements, enables us to work out how many of each atom are contained in a molecule. Compounds which end in “ide” contain 2 different elements. Examples Carbon Hydride Symbol C H Valency 4 1 Swap 1 4 Formula CH4 Magnesium Oxide Symbol Mg O Valency 2 2 Swap 2 2 Formula Mg2O2 which can be simplified to MgO Roman Numerals The valency of transition metal elements can be different, depending on what they are bonded to e.g. Iron can have a valency of 1, 2 or 3! The valency or the element is indicated in the compound name e.g. Iron (I) Chloride, Iron (II) Chloride or Iron (III) Chloride. IV would mean a valency of 4 and V would mean a valency of 5 Iron(I) Chloride Symbol Fe Cl Valency 1 1 Swap 1 1 Formula FeCl Iron(II) Chloride Symbol Fe Cl Valency 2 1 Swap 1 2 Formula FeCl2 Group Ions In some cases, we can have a number of atoms joining together to form a group ion e.g. A sulphur and 4 oxygens combine to form a sulphate ion SO4 (see p4 of data book). These group ions have a charge. Since the charge is caused by gaining or losing electrons, then the size of the charge also tells us the number of bonds the ions form. SO42- would form 2 bonds, whereas NO3- would form 1 bond When working out the formula of compounds containing these groups, it is important that we do not change the no. of atoms in the group. e.g. SO4 is sulphate but SO3 is sulphite If a compound ends in “ate” or “ite” you must find the group ion in the data book We treat the group like it is in a bubble, we do not change its formula Sodium Nitrate Symbol Na NO3 NO3 Valency 1 1 Swap 1 1 Formula NaNO3 Aluminium Sulphate Symbol Al SO4 Valency 3 2 Swap 2 3 Formula Al2 SO4 3 Its looks silly having it in a bubble, so if we have more than one of the ions then we use brackets e.g. Al2(SO4)3 Ionic Formula Ionic compounds are made up of charged particles. It is sometimes necessary to show the charges on the ions which make up an ionic compound. Metal ions have a positive charge, since they are formed when a metal atom loses negatively charged electrons. Non-metal ions have a negative charge since they are formed when a non-metal atom gains negatively charged electrons. To show the ionic formula of a compound, first we must establish its chemical formula. Sodium Chloride Symbol Na Cl Valency 1 1 Swap 1 1 NaCl Formula We then put in the charge for each ion. Sodium is a metal, so has a positive charge. It has a valency of 1, therefore it will have a 1+ charge i.e. Na+ Chlorine is a non-metal, so it has a negative charge. It has a valency of 1, therefore it will have a 1- charge i.e. ClTherefore the ionic formula of sodium chloride is Na+ClNote that the charges balance each other out, so overall the compound is neutral. Even though the charges balance each other out, we must still show them. Iron(II) Chloride Symbol Fe Cl Valency 2 1 Swap 1 2 Formula FeCl2 The roman numeral tells us that iron has a valency of 2, therefore it has a 2+ charge. Chloride ions as we saw above, have a 1- charge. So the ionic formula for iron(II) chloride would be Fe2+ (Cl-)2 Note: If we have more than one ion, we must put the symbol and charge in brackets. The 2 chloride ions with their 1- charges, balances the 2+ on the iron. Meaningful Names Some compounds do not follow the valency rules, but working out the chemical formula of these compounds is much easier than using the valency. We can work out the formula by looking at the name of the compound. Carbon Monoxide DiNitrogen Oxide Phosphorus TriChloride Carbon TetraChloride Nickel PentaIodide Uranium HexaFluoride The prefixes tell us the no. of atoms present. Mono – 1, Di – 2, Tri – 3, Tetra – 4, Penta – 5, Hexa – 6 If a compound name contains one of these prefixes, then valency does not apply Carbon Monoxide The name tells us there is 1 Oxygen and if no prefix is given for the other element, we assume that there is 1 atom. i.e. CO DiNitrogen Oxide The name tells use there are 2 Nitrogens and there is no prefix on the oxide, so we assume that there is 1 oxygen i.e. N2O Work out the rest for yourself. Work out the formula for the following compounds: Calcium Fluoride Nitrogen Hydride Hydrogen Sulphide Lithium Nitrate Sodium Carbonate Magnesium Sulphate Silver(I) Iodide Copper(II) Chloride Iron(II) Nitrate Work out the ionic formula for the following compounds: Potassium Chloride Sodium Sulphide Aluminium Oxide Calcium Hydroxide Iron(III) Sulphite Potassium Phosphate