Unit 1: Inorganic Chemistry
advertisement
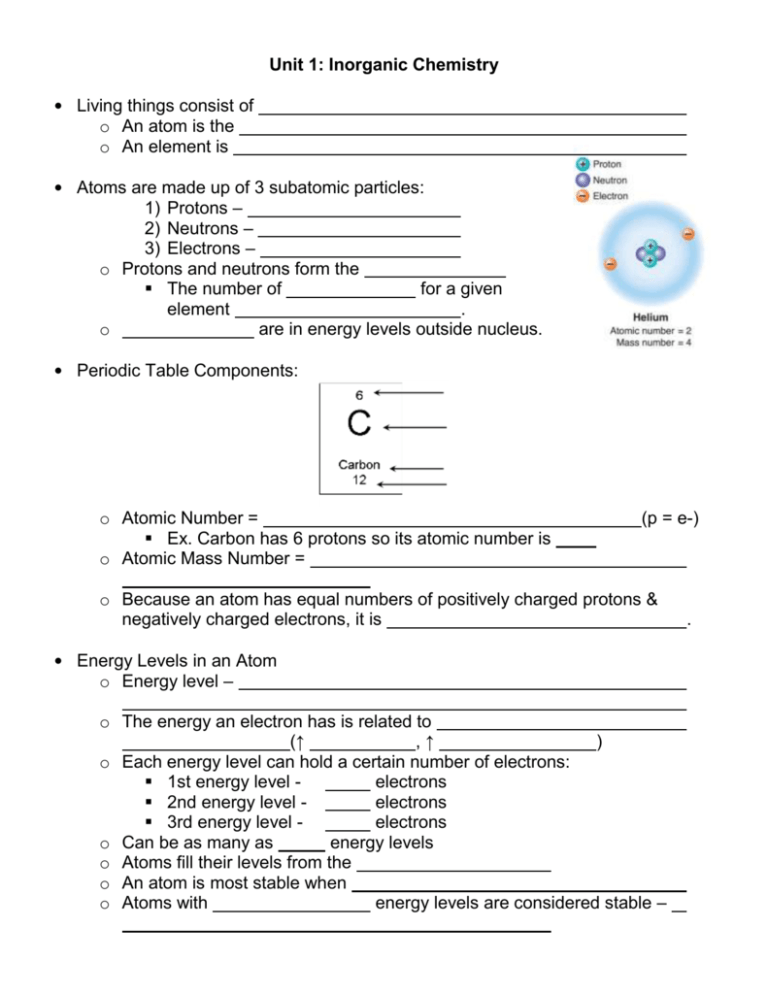
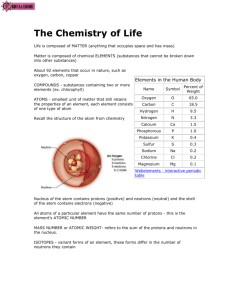
Unit 1: Inorganic Chemistry • Living things consist of o An atom is the o An element is • Atoms are made up of 3 subatomic particles: 1) Protons – 2) Neutrons – 3) Electrons – o Protons and neutrons form the The number of for a given element . o are in energy levels outside nucleus. • Periodic Table Components: o Atomic Number = Ex. Carbon has 6 protons so its atomic number is o Atomic Mass Number = (p = e-) o Because an atom has equal numbers of positively charged protons & negatively charged electrons, it is • Energy Levels in an Atom o Energy level – o The energy an electron has is related to (↑ ,↑ ) o Each energy level can hold a certain number of electrons: 1st energy level electrons 2nd energy level electrons 3rd energy level electrons o Can be as many as energy levels o Atoms fill their levels from the o An atom is most stable when o Atoms with energy levels are considered stable – . o Atoms tend to interact in ways that • A compound is made of . o Examples: water (H2O), carbon dioxide (CO2) o Chemical bonds o 2 Types of Chemical Bonds: 1) Ionic Bonds • An ion is • An ion forms because an atom is more stable when its outermost energy level is full • An atom with few electrons in its outer energy level tends to electrons & those with a nearly full outer energy level tends to electrons. • positive ions • negative ions • Ionic bonds . 2) Covalent Bonds • A covalent bond forms when atoms . • Covalent bonds are • A molecule is Compounds that Dissolve in Water o When one compound dissolves in another a o A solution has 2 parts: Solvent Solute o The amount of solute dissolved in a certain amount of solvent is a Some compounds break up into ions when they dissolve in water: o An Acid is a compound that: Releases a proton – a hydrogen ion (H+) – when dissolved in water The acid increases o A Base is a compound that: Remove H+ ions from a solution The base decreases o A solution’s acidity or H+ concentration is measured by the pH scale is usually 0 to 14 – A solution with a pH of 0 is – A solution with a pH of 14 is – A solution with a pH of 7 is