Carbon Steel - LearnEASY.info
advertisement

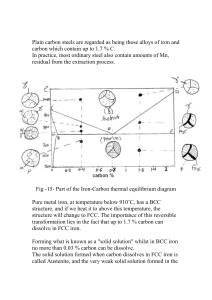
Section 4. Steel Did you know? Iron is abundant in the universe, found in the sun and many types of stars in considerable quantity. The core of the earth is thought to be made up of nickel and iron, and is hotter than the Sun's surface. This intense heat from the inner core causes material in the outer core and mantle to move around (convection currents). Carbon Steel Steel is an alloy of iron (Fe) and carbon (C), with 0.2 to 2.04% carbon by weight. Carbon is the most cost-effective alloying material for iron, but various other alloying elements are used such as manganese, chromium, vanadium, and tungsten. Carbon and other elements act as a hardening agent, preventing dislocations in the iron atom crystal lattice from sliding past one another. Carbon Steel ANSI def'n General Def'n Low carbon steel 0.05–0.15% <0.1% Mild Steel 0.16-0.29% 0.1-0.25% Low tensile strength, but it is cheap and malleable; surface hardness can be increased through carburizing. Medium carbon steel 0.30–0.59% 0.25-0.45% Balances ductility and strength and has good wear resistance; used for large parts, forging and automotive components. High carbon steel 0.6–0.99% 0.45-1.0% Very strong, used for springs and high-strength wires. 1.0–2.0% 1.0-1.50% (>1.5% rare) Very hard - knives, punches. Usually anything over 1.2% would be made with powder metallurgy and is considered a high alloy carbon steels. – 2.5-4.0% Ultra-high carbon steel Cast Iron Applications and properties Soft, ductile. Easy to form. Lower melting point, easy casting, lower toughness and strength than steel. Varying the amount of alloying elements and the way they incorporated into the steel (solute elements, precipitated phase) influences such properties as hardness, ductility and tensile strength of the resulting steel. With increased carbon content steel becomes harder and stronger than iron, but also more brittle. The maximum solubility of carbon in iron (in austenite region) is 2.14% by weight, occurring at 1149 °C; higher concentrations of carbon or lower temperatures will produce cementite (very brittle). Add any more carbon and you get cast iron, which has a lower melting point and is easier to cast. Wrought iron containing only a very small amount of other elements, but contains 1–3% by weight of slag in the form of particles elongated in one direction, giving the iron a characteristic grain. It is more rust-resistant than steel and welds more easily. It is common today to talk about 'the iron and steel industry' as if it were a single entity, but historically they were separate products. Steel has been produced for thousands of years, but it became common after more efficient production methods were devised in the 17th century. The Bessemer process in the mid 1800's made steel relatively inexpensive for mass-produced goods. Further refinements in the process, such as basic oxygen steelmaking, further lowered the cost of production while increasing the quality of the metal. Today, steel is one of the most common materials in the world and is a major component in buildings, tools, automobiles, and appliances. Page 1 of 4 Hardening of Steel Temper colours can be used as a guide to temperature. Alloys such as stainless steel form thinner films than do carbon steels for a given temperature and hence produce a colour lower in the series. For example, pale straw corresponds to 300°C for SS, instead of 230°C for CS. Temper Colour Temp °C Examples applications Pale straw 230 Planing and slotting tools Dark straw 240 Milling cutters, drills Brown 250 Taps, shear blades for metals Brownish-purple 260 Punches, cups, snaps, twist drills, reamers Purple 270 Press tools, axes Dark purple 280 Cold chisels, setts for steel Blue 300 Saws for wood, springs Dark Blue 450-650 Toughening for constructional steels VIDEO: Properties and Grain Structure. BBC 1973 (These are available on the network, but not on the website) Part 1: What is a grain? (Video) 1. The patches seen on a galvanised object are crystals or grains of zinc. 2. All metals are made up of grains, but they are usually invisible (too small to see or same shine/colour). 3. Etching Process: Mirror finish, powerful acid, washed and sealed. 4. In a pure metal, the grains are different colours because of the way they reflect the light. 5. Tiny crystals grow outward until they meet. Each fully grown crystal is called a grain. Part 2: Recrystallisation (Video) 1. Before cold working the grains are similar size and shape 2. Cold working elongates the grains, increases hardness and strength increases, reduces ductility. 4. At 350C, new grains form in the Al to replace old grains. Called recrystallisation 5. Recrystallisation softens, lowers the strength, ductility increased 6. Excessive recrystallisation temp gives poor mechanical properties Part 3: Heat Treatment of Steel (Video) 1. Steel grains are too small to be visible - need a microscope approx 250 times magnification. 2. Ferrite: Light coloured. Made of iron. Ductility to the steel 3. Pearlite: darker coloured. Layers of Iron + Iron Carbide. Hardess and strength to the steel 4. 100% Pearlite is about 0.8%C. Pearlite, recrystallisation temperature 720C. 5. Normalising - cooled in air, reduced grain size and more uniform shape, toughness increased 6. Quenching - increases hardness. Not enough time for pearlite to form, so a needlelike strcture forms martensite. Very hard and brittle. 7. Tempering - (after quenching) restores toughness. Modifies the martensite needles with small flakes of carbon. This gives hardness AND toughness. 8. 0.1%C steel (Mild Steel). Recrystalisation 900C. Not enough carbon to produce martensite. Iron-Carbon Equilibrium Diagram An Equilibrium diagram is a graph of the different structural arrangements that occur within a range of an alloying element. Page 2 of 4 This diagram shows how iron and carbon combines IF it is cooled slowly (in equilibrium). Under 2% is steel, over 2% is cast iron. Cementite Fe3C has 6.67%C and it is basically a ceramic. The eutectoid (pearlite) at E has 0.83% C, less carbon is a hypoeutectoid steel (A), and greater is hypereutectoid (B). Alpha iron (ferrite), gamma iron (austenite, which only exists at high temperature), and delta iron (another high temp structure). Allotropic changes take place when there is a change in crystal lattice structure. From 1539º-1400ºC the delta iron has a body-centered cubic lattice structure. At 1400ºC, the lattice changes from a body-centered cubic to a face-centered cubic lattice type. At 210ºC, the curve shows a plateau but this does not signify an allotropic change. It is called the Curie temperature, where the metal changes its magnetic properties. Two very important phase changes take place at 0.83%C and at 4.3% C. At 0.83%C, the transformation is eutectoid, called pearlite. These 2 phases separate out in layers. gamma (austenite) --> alpha + Fe3C (cementite) At 4.3% C and 2066ºF, the transformation is eutectic, called ledeburite. L(liquid) --> gamma (austenite) + Fe3C (cementite) This is way too much carbon for steel. Alloy Steels Effects of alloying elements on tool steel properties: Carbon: Raising carbon content increases hardness slightly and wear resistance considerably. Dramatic increases to hardness & strength when heat treated. Manganese: Small amounts of of Manganense reduce brittleness and improve forgeability. Larger amounts of manganese improve hardenability, permit oil quenching, and reduce quenching deformation. Silicon: Improves strength, toughness, and shock resistance. Page 3 of 4 Tungsten: Improves "hot hardness" - used in high-speed tool steel. Vanadium: Refines carbide structure and improves forgeability, also improving hardness and wear resistance. Molybdenum: Improves deep hardening, toughness, and in larger amounts, "hot hardness". Used in high speed tool steel because it's cheaper than tungsten. Chromium: Improves hardenability, wear resistance and toughness. Nickel: Improves toughness and wear resistance to a lesser degree. Including these elements in varying combinations can act synergistically, increasing the effects of using them alone. Tool Steels Tool steels are covered in Australian Standard AS1239 and is virtually the same as the American AISI tool steel classification. (Similarly with British Standard 4659) For example: AS 1239 grade H13 hot work tool steel containing 0.35% carbon, 5.0% chromium, 1.5% molybdenum and 1% vanadium would be written as X40CrMoV51 in DIN (German). High Speed Steels, for example: AS 1239 grade M2 Containing 0.85% carbon, 4.0% chromium, 5.0% molybdenum, 6.0% tungsten, 2.0% vanadium would be written as S 6-5-2 in DIN. Glossary 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. Alloy: A metallic substance that is composed of two or more elements. Austenite: Face-centered cubic iron or an iron alloy based on this structure. Bainite: The product of the final transformation of austenite decomposition. Body-centered: A structure in which every atom is surrounded by eight adjacent atoms, whether the atom is located at a corner or at the center of a unit cell. Cementite: The second phase formed when carbon is in excess of the solubility limit. Critical point: Point where the densities of liquid and vapor become equal and the interface between the two vanishes. Above this point, only one phase can exist. Delta iron: The body-centered cubic phase which results when austenite is no longer the most stable form of iron. Exists between 2802 and 2552 degrees F, has BCC lattice structure and is magnetic. Eutectic: A eutectic system occurs when a liquid phase tramsforms directly to a two-phase solid. Eutectoid: A eutectoid system occurs when a singlephase solid transforms directly to a two-phase solid. Face-centered: A structure in which there is an atom at the corner of each unit cell and one in the center of each face, but no atom in the center of the cube. Ferrite: Body-centered cubic iron or an iron alloy based on this structure. Fine pearlite:Results from thin lamellae when cooling rates are accelerated and diffusion is limited to shorter distances. Hypereutectoid: Hypereutectoid systems exist below the eutectoid temperature. Hypoeutectoid: Hypoeutectoid systems exist above the eutectoid temperature. 15. Ledeburite: Eutectic of cast iron. It exists when the carbon content is greater than 2 percent. It contains 4.3 percent carbon in combination with iron. 16. Liquidus Line: On a binary phase diagram, that line or boundary separating liquid and liquid + solid phase regions. For an alloy, the liquidus temperature is that temperature at which a solid phase first forms under conditions of equilibrium cooling. 17. Martensite: An unstable polymorphic phase of iron which forms at temperatures below the eutectoid because the face-centered cubic structure of austenite becomes unstable. It changes spontaneously to a bodycentered structure by shearing action, not diffusion. 18. Microstructure: Structure of the phases in a material. Can only be seen with an optical or electron mircoscope. 19. Pearlite: A lamellar mixture of ferrite and carbide formed by decomposing austenite of eutectoid composition. 20. Phase: A homogeneous portion of a system that has uniform physical and chemical characteristics. 21. Phase diagram: A graphical representation of the relationships between environmental constraints, composition, and regions of phase stability, ordinarily under conditions of equilibrium. 22. Polymorphic: The ability of a solid material to exist in more than one form or crystal structure. 23. Quench: To rapidly cool 24. Solidus Line: On a phase diagram, the locus of points at which solidification is complete upon equilibrium cooling, or at which melting begins upon equilibrium heating. 25. Solubility: The amount of substance that will dissolve in a given amount of another substance. Page 4 of 4