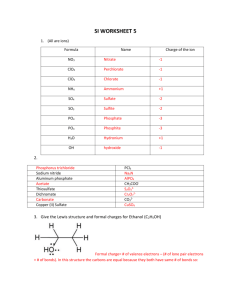
CHM 234: Worksheet #1
advertisement

CHM 234: Worksheet #10 Chapter 7 Spring 2011 Dr. Halligan Resonance Concepts Electrons can be moved in one of the following ways: 1. Move pi electrons toward a positive charge or toward a pi bond. 2. Move lone pair electrons toward a pi bond. 3. Move a single nonbonding electron toward a pi bond. Remember the two important “resonance commandments. 1. Thou shalt not break a single bond. 2. Thou shalt not violate the octet rule. When you draw the arrows, you must begin at the source of electrons. Here are some questions you should ask yourself when drawing resonance structures: a. Can we convert any lone pairs into pi bonds without violating the two commandments? b. Can we convert any pi bonds into lone pairs without violating the two commandments? c. Can we convert any pi bonds into pi bonds without violating the two commandments? Are you starting to recognize patterns? Here is a list of five patterns that you should learn to recognize and you should become proficient at drawing resonance structures for these cases. a. b. c. d. e. A lone pair next to a pi bond. A lone pair next to a positive charge. A pi bond next to a positive charge. A pi bond between two atoms, where one of those atoms is electronegative. Pi bonds going all the way around a ring. Worksheet #10 Questions 1. Draw resonance contributors for the following ions: a b c 2. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which species are major contributors and which are minor contributors to the resonance hybrid. O a b C N O c H d 3. Which resonance contributor makes the greatest contribution to the resonance hybrid? Justify your answer. a or O O or b 4. Which species is more stable? Justify your answer. O a b O or or c H N or O O d O or 5. Draw the MO diagram for 1,3,5,7-octatetraene and answer the following questions. a. How many MOs does the compound have? b. Which are the bonding MO’s and which are the antibonding MOs? c. Which MOs are symmetric and which MOs are antisymmetric? d. Which MO is the HOMO and which is the LUMO in the ground state? e. Which MO is the HOMO and which MO is the LUMO in the excited state? f. How many nodes does the highest-energy MO have? 6. For the reaction of 1-propenyl-1-cyclohexene with HCl, (1) give the major 1,2- and 1,4-addition products and (2) indicate which is the kinetic product and which is the thermodynamic product. Explain your answer. 7. Write a mechanism for the following Diels-Alder reactions. O + a b O + HO O O c + H H COOH + d HOOC H 8. What diene and what dienophile were used to synthesize the following compounds? Hint: write a mechanism for the “retro-Diels-Alder” reaction. O b a O O O NO2 c H H d O