Example questions for Chapter 15
advertisement

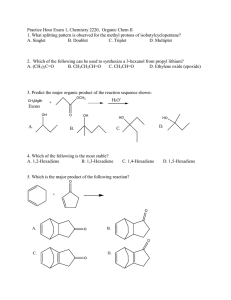
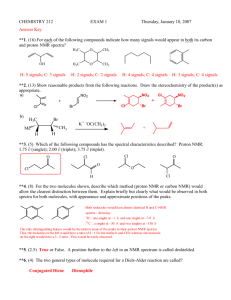
Example questions for Chapter 15 (Note: You need a table of 1 H-NMR and 13 C-NMR chemical shifts (from your textbook) to answer these questions.) 1. Explain the principle of an NMR experiment: include the effect of spin orientation in a magnetic field, the type of transition that corresponds to NMR absorption, how this transition is induced, and what kind of frequency is used to induce the transition. 2. Making using of a drawing, explain the shielding of a nucleus by its surrounding electrons. 3. The 1 H NMR and the IR spectra of a compound with molecular formula C4 H8 O are shown below. In the 1 H-NMR spectrum, the integration numbers (number of protons that give rise to that peak) are 3, 3, and 2, for the peaks at δ ≈ 1.0, 2.1, and 2.5 ppm, respectively. (a) What is the structure of the compound? (b) Explain the NMR spectrum. (c) What information did the IR spectrum give you? (d) Sketch the 13 C NMR spectrum of this compound. 1 4. The 13 C-NMR spectrum of 2,2,3-tribromobutane is shown below. (a) Identify each peak as best as you can. Suggestion: draw the structure of 2,2,3-tribromobutane, number the carbons, and write those numbers on the spectrum, over the corresponding peaks (for example, C1,...). (b) How many equivalent protons do you expect in the 1 H-NMR spectrum of this compound? (c) Sketch the 1 H NMR spectrum of 2,2,3-tribromobutane. 5. Indicate how many types of equivalent protons you expect in the 1 H-NMR spectrum and how many types of equivalent carbons in the 13 C-NMR spectrum of the following compounds. For the protons, indicate the multiplicity expected (if more than a quartet, indicate ‘complex ’multiplet). CH3 OH CH3 CH3 2 6. The 1 H NMR spectra of two compounds with chemical formula C3 H7 Cl are shown below. Note: the multiplicities are not clear in these two spectra, but are indicated here. For the top spectrum, the integration numbers (I) and multiplicities are as follows: δ=1.1 (I=6, doublet), δ=3.8 (I=1, septet). For the bottom spectrum, the integration numbers and multiplicities are as follows: δ=0.9 (I=3, triplet), δ=1.7 (I=2, complex multiplet), δ=3.4 (I=2, triplet). The two compounds are constitutional isomers. Propose a structure for each of the compounds and explain your reasoning. 3 7. The IR and 13 C NMR spectra of an organic molecule with formula C3 H6 O are shown below. Propose a structure compatible with these data and explain your reasoning. 4 8. The IR and 1 H NMR spectra of a compound with chemical formula C2 H6 O are shown below. The integration numbers (int) and multiplicities (multip) are as follows: δ=1.2 (I=3, triplet), δ=2.6 (I=1, singlet; note: this peak has a very variable chemical shift), δ=3.7 (I=2, quartet). Propose a structure compatible with these data and explain your reasoning. 9. BONUS QUESTION: name the following compounds OH O O C C CH3 CH3CH2CH3 CH3 CH3 CH3CH2CH2CH3 CH3 CH3CH2OH 5 OH CH3OH HO C CH3 CH3CH3 CH3CH2OCH2CH3 CH3