Practice Hour Exam 1, Chemistry 2220, Organic Chem II
advertisement
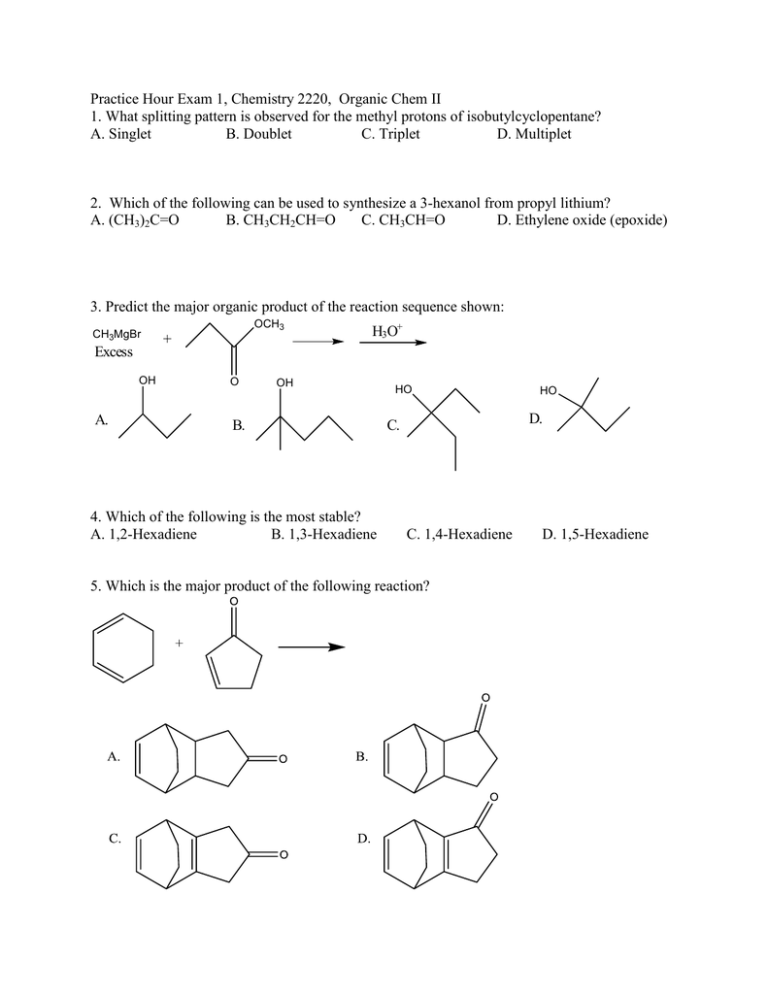
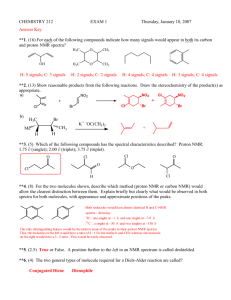
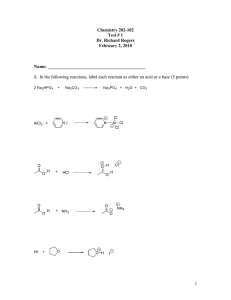
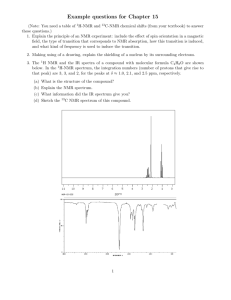
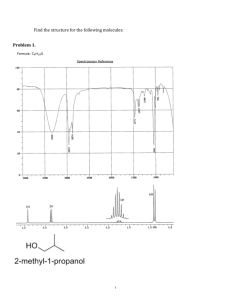
Practice Hour Exam 1, Chemistry 2220, Organic Chem II 1. What splitting pattern is observed for the methyl protons of isobutylcyclopentane? A. Singlet B. Doublet C. Triplet D. Multiplet 2. Which of the following can be used to synthesize a 3-hexanol from propyl lithium? A. (CH3)2C=O B. CH3CH2CH=O C. CH3CH=O D. Ethylene oxide (epoxide) 3. Predict the major organic product of the reaction sequence shown: CH3MgBr Excess OH A. OCH3 + O H3O+ OH B. 4. Which of the following is the most stable? A. 1,2-Hexadiene B. 1,3-Hexadiene HO HO D. C. C. 1,4-Hexadiene 5. Which is the major product of the following reaction? D. 1,5-Hexadiene 6. Which of the following reactions can be used in the synthesis of 5-bromo-1,3-cyclohexadiene from 1,3-cyclohexadiene? A. Br2/cold, dark B. HBr C. HBr/peroxides D. Br2/heat 7. What feature would you expect to see in the proton NMR spectrum of product B after subjecting A to the following reaction? A. B. C. D. There will be no signals with chemical shift greater than 4 ppm The integration of the alkyl protons (region 1-3 ppm) will be 8 H The signal for the methyl group will be a triplet The number of signals in the spectrum will increase 8. Which of the following compounds, all of molecular formula C7H12O2, would give proton NMR spectrum that consists of two singlets only? O O A. B. O O O C. D. O O O 9. Which of the following gives no reaction? 10. Which of these is NOT a product for the following reaction? HI 1 mole I A. B. I I C. D. I 11. Which hydrogen atom in the following molecule is most susceptible to abstraction by free radicals? H H C. A. H C H B. H D. H 12. An unknown compound gave a 1H NMR spectrum consisting only of a doublet at δ0.91 (6H), a multiplet at δ1.53 (1H), a multiplet at δ1.62 (2H) and a triplet at δ3.68 (2H). The most likely structure is: 13. An unknown compound gave a 1H NMR spectrum consisting only of a singlet at δ2.48 (3H), a multiplet at δ7.54 (4H) and a singlet at δ9.52 (1H). The most likely structure is: 14. What is the final product? 15. An unknown compound does not react with H2CrO4 but does react with cold KMnO4. Which of these is most likely the compound? 16. What is the product of the following reaction? 17. Which diene and dienophile would you use to synthesize the following compound? 18. omit 19. What product(s) form when CH3Li reacts with CH3CO2H, then H+? A. (CH3)2C(OH)2 B. (CH3)3COH C. (CH3)2C=O D. CH4 + CH3CO2H 20. What is the m/z for the molecular ion of CH3CH2CH3? C=12, H=1 A. 3 B. 15 C. 29 D. 44 21. What statement about Diels-Alder reactions is false? A. The diene must be able to adopt the s-cis conformation. B. The diene must be conjugated. C. There is a kinetic preference for the exo product isomer. D. The reaction works best when the diene is electron rich and the dienophile is electron poor, or vice versa. 22. What is the main product of this transformation? A. CH3CH2CH2CH(OH)CH3 B. CH3CH2CH2CH2CH2OH C. (CH3)2CHCH(OH)CH3 D. (CH3)2CHCH2CH2OH 23. What is the main product of this reaction? A. B. C. D. 24. Which of the following structures is a resonance structure of the Lewis structure shown here? A. B. C. D. 25. In the 1H NMR spectrum of CH3CH2OH, the oxygen acts as an electron and the adjacent CH2 group. A. withdrawing, deshields B. withdrawing, shields C. donating, deshields D. donating, shields group