CHE 230 - Organic Chemistry
advertisement

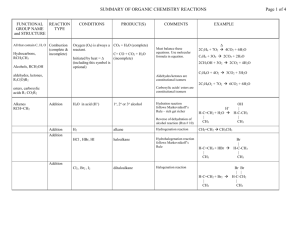
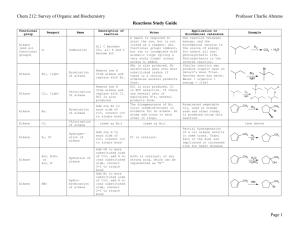
CHE 230 - Organic Chemistry Exam 2, March 12, 2003 Name Student ID No. KEY Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place your student identification on your desk. A proctor will come around to check everyone’s ID. Third: Read through the entire exam. Your goal, as always, is to score as many points as possible. Do not waste time on problems that you can’t do if there are others that look easy. Fourth: It is critically important that your answers be written in a clear, unambiguous manner. Answers in which your intentions are unclear will not receive credit. Fifth: READ THE INSTRUCTIONS FOR EACH PROBLEM. You have until 9:50 to complete this exam. There will be no extensions, so budget your time carefully. If you wish to have your exam score posted beside your student ID number in the glass case (1st floor, CP Building, behind CP-139) with the exam key, place an ‘X’ in this space . If you do not mark this space, your exam score will not be posted. Problem Points 1. 8 2. 10 3. 8 4. 10 5. 4 6. 9 7. 15 8. 10 9. 10 10. 6 11. 10 Total 100 Score 1 1. (8 points) For each pair of structures below, identify them as diastereomers, enantiomers, or identical. SAME DIASTEREOMERS NH2 NH2 ENANTIOMERS SAME 2. (10 points) Circle the best nucleophile in each of the following pairs. Assume that the comparison is being made in polar aprotic solvent. a) H2O or H2S b) NH3 or KNH2 c) NaSCH3 or HSCH3 d) NaI or NaCl e) or NH3 N 2 3. (8 points) What nucleophiles would serve to effect the following conversions of 1iodopropane? Please provide a complete, neutral species (i.e. "NaBr), not "Br-"). Reagent N3 SH reagent I CN O 4. NaN3 NaSH NaCN NaOPh (10 points) Draw each of the following compounds in the most stable chair conformation. Be sure that each carbon with substitutents appears to be tetrahedral. a) CH3 tBu H H3C tBu H H b) CH3 CH3 H H3C H CH3 H 5. H (4 points) Which of the following polycyclics has a boat cyclohexane within it? (circle all that do). H H H H H H 3 6. (9 points) Draw an energy profile diagram for the reaction shown below. On your diagram, label the transition state and the activation energy for the rate-determining step. LiCN CH3CH2Br CH3CH2CN CH3CN Transition state Energy Ea Reaction Progress 7. (15 points) Predict the products of each reaction below. Be sure that you indicate stereochemistry, where relevant. If you believe that no reaction will occur, write "NO REACTION." a) Br NaI, CH3CN No Reaction b) NaI Cl I acetone c) Cl CN NaCN DMF Cl Cl 4 8. (10 points) For each of the reactions below, determine if they proceed by a SN2 or SN1 or mechanism. a) CH3NH2 Br Br N S N2 b) Br OH H2O, heat S N1 S 9. racemic (10 points) Explain with crystal clarity why treatment of propyl alcohol with bromide ion fails to give propyl bromide, but treatment with HBr succeeds. Do not exceed the space provided. A sentence or two will suffice. Na+Br- Br OH HBr Br In the first case, hydroxide would have to be a leaving group, but it ISN'T a leaving group in substitution rxns. In the second case, the hydroxyl gets protonated, so the leaving group is H2O, a fine leaving group. 5 10. (6 points) Draw a viable mechanism for the reaction below. Be sure that you use the mechanism arrows correctly. OTs N(CH3)3 (CH3)3N + OTs N(CH3)3 OTs + OTs N 11. (10 points) Explain why adamantyl bromide (1) fails to give the alcohol product shown, although other tertiary bromides (like 2) react readily. H2O Br OH 1 Br H2O OH fast 2 Dissociation of adamantyl bromide cannot produce a planar sp2 cation, so ionization is unfavorable. The second case does not have this restriction, so formation of the cation intermediate proceeds normally. END OF EXAM 6







![Organic Chemistry I Exam 3 [2008]](http://s3.studylib.net/store/data/008126463_1-17b9ea8b39e09644df3aed1c21943738-300x300.png)