Chemistry 2 test example
advertisement

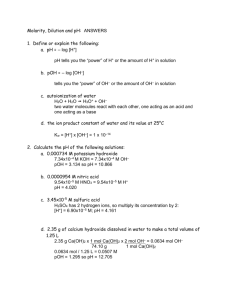
Chemistry 2 Exam Roane State Academic Festival Name______________________________________(print neatly) School _________________ There are fifteen question on this exam. Each question is weighted equally. On the answer sheet, write your name in the space provided and your answers in the blanks provided. There is also a bonus question. It will be used as a tie breaker and will be assigned partial credit. Therefore, for this bonus question show your work VERY NEATLY in the space provided on the answer sheet. When you are finished bring you answer sheets to the front and exit quietly. Please refrain from discussing the exam in the hallway since others may still be working NOTE: If there are units associated with the answer, full credit with NOT be given without the units. 1 mole = 6.022 × 1023 particles 760 torr / 1 atm R = 0.08206 L atm mol-1 K-1 1. How many molecules are there in 52.0 g of NH3? 2. In the following reaction is not balanced. For the real reaction 57.0 g of UH3 are formed. How many grams of hydrogen are needed to produce this 57.0 g of product? U(s) + H2(g) ! UH3(s) 3. What volume of 1.00 M KOH solution would contain 52.0 g of KOH? 4. What volume of 0.1021 M NaOH is required to reach the second endpoint of 25.00 mL of a 0.1300 M H3PO4 solution? The reaction is: 2NaOH + H3PO4 ! Na2HPO4 + 2H2O 5. What is the volume of a gas, originally at 650 torr with a volume of 57.0 L, if it were compressed and the pressure increased to 1500 torr? 6. Calculate the normality of an unknown acid if 0.456 g of NaOH is required to neutralize 20.00 mL of the acid. 7. Balance the following redox reaction: NO2 + H2O ! HNO3 + NO 8. For the following reaction, the pressures listed below the reactants and products are the pressures observed. Calculate the K for the reaction. N2(g) 0.60 atm + 3H2(g) 0.42 atm º 2NH3 0.11 atm 9. Calculate the enthalpy of the following reaction: 2C2H5OH(l) + 7O2(g) ! 4CO2(g) + 6H2O(g) ∆fHO(C2H5OH(l)) = !277.7 kJ mol!1 ∆fHO(CO2(g)) = !393.51 kJ mol!1 ∆fHO(H2O(g)) = !241.8 kJ mol!1 10. For the following reaction tell what would happen if one were to 1) increase the Cl2 pressure, 2) increase the overall pressure and 3) increase the temperature. PCl3 + Cl2 º PCl5 ∆rHO = !108 kJ mol!1 11) What is the molar solubility of AgBr in pure water? The Ksp for AgBr is 5.35 × 10-13. 12) How many grams of calcium fluoride, CaF2, can dissolve in a solution that contains 0.010 M sodium fluoride, NaF? (Ksp (CaF2) = 4.0 × 10!11) 13) What is the pH of a solution that has a total OH! concentration of 2.5 × 10-8? 14) What is the pH of a 0.10 M solution of HCOOH. (Ka (HCOOH) = 1.76 × 10!4) 15) Using the standard potential tables, determine the potential for the following reaction: 2PbSO4 + 2H2O ! Pb + PbO2 + 2H2SO4 Chemistry 2 Exam Roane State Academic Festival Answer Sheet Team _______________________ Name_________________________________________ (PRINT NEATLY) School ______________________ 1. __________________________________________________________________________ 2. __________________________________________________________________________ 3. __________________________________________________________________________ 4. __________________________________________________________________________ 5. __________________________________________________________________________ 6. __________________________________________________________________________ 7. __________________________________________________________________________ 8. __________________________________________________________________________ 9. __________________________________________________________________________ 10. 1) The reaction shifts to the _________________________________ 2) The reaction shifts to the _________________________________ 3) The reaction shifts to the _________________________________ 11. __________________________________________________________________________ 12. __________________________________________________________________________ 13. __________________________________________________________________________ 14. __________________________________________________________________________ 15. __________________________________________________________________________ Bonus Question answer:_________________________________________________________ Chemistry 2 Exam Roane State Academic Festival Bonus Question Name______________________________________(print neatly) School _________________ Answer the following question showing all your work NEATLY. In a titration of acetic acid (CH3COOH) with NaOH, the pH of the solution was measured when 10.00 mL of NaOH was added to 25.00 mL of CH3COOH. The concentration of the NaOH was 0.1412 M and the concentration of the CH3COOH was 0.1054 M . What was the pH? (Ka for CH3COOH is 1.75 × 10–5) Give your answer to the nearest 0.01 pH unit. Periodic Chart of the Elements 1 2 3 4 5 6 7 8 9 10 11 12 ²s electronsÿ 13 14 15 16 17 18 ²!!!!!!!!!!!p electrons!!!!!!!!!!!ÿ 1.008 1H 4.002 2He ²!!!!!!!!!!!!!!!!!!!!!!!!!!d electrons!!!!!!!!!!!!!!!!!!!!!!!ÿ 6.941 3Li 9.012 4Be 10.81 5B 12.01 6C 14.01 7N 16.00 8O 19.00 9F 20.18 10Ne 22.99 11Na 24.31 12M g 26.98 13Al 28.09 14Si 30.97 15P 32.07 16S 35.45 17Cl 39.95 18Ar 39.10 19K 40.08 20Ca 44.96 21Sc 47.88 22Ti 50.94 23V 52.00 24Cr 54.94 25M n 55.85 26Fe 58.94 27Co 58.69 28Ni 63.55 29Cu 65.39 30Zn 69.72 31Ga 72.61 32Ge 74.92 33As 78.96 34Se 79.90 35Br 83.80 36Kr 85.47 37Rb 87.62 38Sr 88.91 39Y 91.22 40Zr 92.91 41Nb 95.94 42M o 43Tc 101.1 44Ru 102.9 45Rh 105.4 46Pd 107.9 47Ag 112.4 48Cd 114.8 49In 118.7 50 Sn 121.8 51Sb 127.6 52Te 126.9 53I 131.3 54Xe 132.9 55Cs 137.3 56Ba * 5770 175.0 71Lu 178.5 72Hf 180.9 73Ta 183.9 74W 186.2 75Re 192.2 76Ir 190.2 77Os 195.1 78Pt 197.0 79Au 200.6 80Hg 204.4 81Tl 207.2 82Pb 209.0 83Bi 84Po 85At 86Rn 87Fr 88Ra **89102 103Lr 104Rf 105Db 106Sg 107Bh 108Hs 109M t 110Ds ²!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!f electrons!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!ÿ The Lanthanide and Actinide Series (4f and 5f) * º 4f 138.9 57La 140.1 58Ce 140.9 59Pr 144.2 60Nd 61Pm 154.4 62Sm 152.0 63Eu 157.3 64Gd 158.9 65Tb 162.5 66Dy 164.9 67Ho 167.3 68Er 168.9 69Tm 173.0 70Yb ** º 5f 89Ac 232.0 90Th 91Pa 238.0 92U 93Np 94Pu 95Am 96Cm 97Bk 98Cf 99Es 100Fm 101M d 102No The EMF Series - Standard Potentials The Electromotive Series (Redox Potentials). Standard Potential ‡ E» (volts) Li+ + e- W Li(s) -3.045 + K + e W K(s) -2.924 2+ Ca + 2e W Ca(s) -2.76 + Na + e W Na(s) -2.7109 3+ Al + 3e W Al(s) -1.706 Mn2+ + 2e- W Mn(s) -1.029 Cd(OH)2(s) + 2e- W Cd(s) + 2OH-0.812 2+ Zn + 2e W Zn(s) -0.7628 3+ Cr + 3e W Cr(s) -0.74 2+ Fe + 2e W Fe(s) -0.409 2PbSO4(s) + 2e W Pb(s) + SO4 -0.356 2+ Ni + 2e W Ni(s) -0.23 2+ Sn + 2e W Sn(s) -0.1364 2+ Pb + 2e W Pb(s) -0.1263 2H+ + 2e- W H2(g) 0.0000000 4+ 2+ Sn + 2e W Sn +0.15 IO3 + 2H2O +4e W IO + 4OH +0.15 2+ SO4 + 4H + 2e W H2SO3 + H2O +0.20 2+ Cu + 2e W Cu(s) +0.3419 O2(g) + 2H2O(l) + 4e W 4OH +0.401 IO + H2O + 2e W I + 2OH +0.485 NiO2(s) + 2H2O + 2e W Ni(OH)2(s) + 2OH +0.49 I2(s) + 2e- W 2I+0.535 3+ 2+ Fe + e W Fe +0.770 + Ag + e W Ag(s) +0.7996 ClO + H2O(l) + 2e W Cl + 2OH +0.90 + NO3 + 4H + 3e W NO(g) + 2H2O(l) +0.96 Br2(l) + 2e W 2Br +1.065 + O2(g) + 4H + 4e W 2H2O(l) +1.229 Cl2 + 2e W 2Cl +1.3583 + 2+ MnO4 + 8H + 5e W Mn + 4H2O +1.507 + Au + e W Au(s) +1.68 PbO2(s) + 4H+ + SO42- + 2e- W PbSO4(s) + 2H2O(l) +1.685 F2(g) + 2e W 2F +2.87 Reduction Reaction* 6 Oxidation Reaction 7 Notes: All ions are 1.0 M aqueous. (g) = gas at 1 atmosphere. (s) = solid and (l) = liquid. *Reversing the reaction and changing the sign of the potential gives the oxidation reaction and the oxidation potential. ‡ The international convention of the reduction potential as the standard is followed. Source: CRC Handbook of Chemistry and Physics, 54th Edition, CRC Press, Inc. Boca Raton, FL, p D120. The EMF Series - Standard Potentials Chemistry 2 Exam Roane State Academic Festival Answer Sheet Team _______________________ Name_________________________________________ (PRINT NEATLY) School ______________________ 1. _______1.84 × 1024______________________ 2. _______0.355 g_________________________ 3. _______0.927 L_________________________ 4. _______63.66 mL_______________________ 5. ______24.7 L___________________________ 6. _______0.570 N________________________ 7. _______3NO2 ! 2 HNO3 + NO____________ 8. _______2.7 × 10–1 (0.27)______________ 9. _______–2469.4 kJ mol–1_________________ 10. 1) The reaction shifts to the ___right_______ 2) The reaction shifts to the ___right_______ 3) The reaction shifts to the ___left________ 11. _____7.3 × 10–7________________________ 12. ____3.1 × 10–5_________________________ 13. ____6.40_____________________________ 14. ____2.38_____________________________ 15. ____–2.041 V_________________________ Bonus: __4.82 ( the 1:1 buffer is 4.76 )________ partial credit if the got to a reasonable distance: Starting amounts: mmoles of NaOH 1.412 mmol of CH3COOH 2.635 mmol Ending amounts: mmoles of NaCH3OO 1.412 mmol of CH3COOH 1.223 mmol