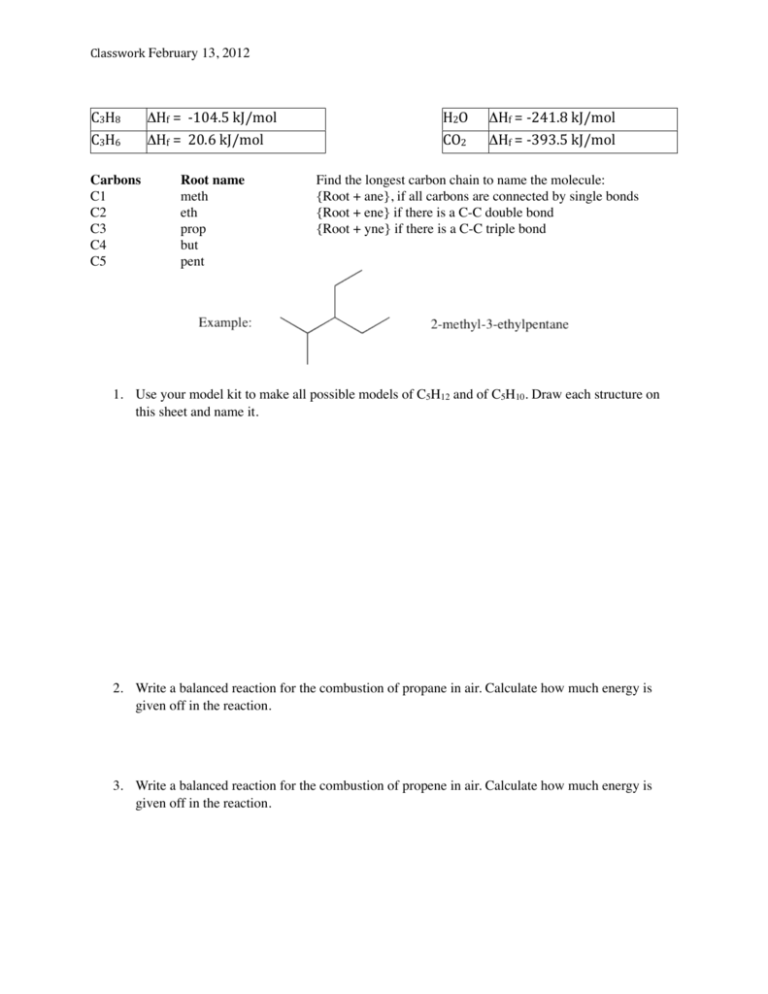
C3H8 AHf = -‐104.5 kJ/mol H2O AHf =
advertisement
Classwork February 13, 2012
C3H8
C3H6
Carbons
C1
C2
C3
C4
C5
ΔHf = -­‐104.5 kJ/mol
ΔHf = 20.6 kJ/mol
Root name
meth
eth
prop
but
pent
H2O
CO2
ΔHf = -­‐241.8 kJ/mol
ΔHf = -­‐393.5 kJ/mol
Find the longest carbon chain to name the molecule:
{Root + ane}, if all carbons are connected by single bonds
{Root + ene} if there is a C-C double bond
{Root + yne} if there is a C-C triple bond
1. Use your model kit to make all possible models of C5H12 and of C5H10. Draw each structure on
this sheet and name it.
2. Write a balanced reaction for the combustion of propane in air. Calculate how much energy is
given off in the reaction.
3. Write a balanced reaction for the combustion of propene in air. Calculate how much energy is
given off in the reaction.